Chapter: Basic & Clinical Pharmacology : Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics,& Drugs Used in Gout
Nonselective Cox Inhibitors
NONSELECTIVE COX INHIBITORS
Diclofenac
Diclofenac is a
phenylacetic acid derivative that is relatively non-selective as a COX
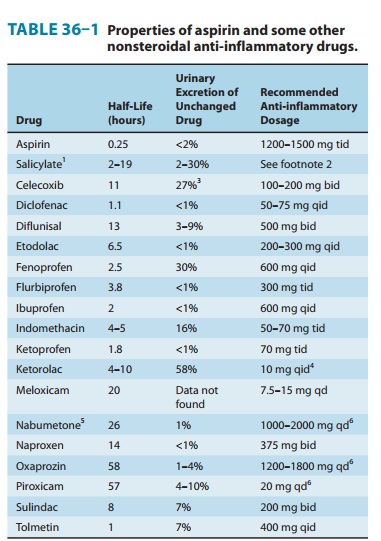
inhibitor. Pharmacokinetic and dosage charac-teristics are set forth in Table
36–1.
Gastrointestinal
ulceration may occur less frequently than with some other NSAIDs. A preparation
combining diclofenac and misoprostol decreases upper gastrointestinal
ulceration but may result in diarrhea. Another combination of diclofenac and
omeprazole was also effective with respect to the prevention of recurrent
bleeding, but renal adverse effects were common in high-risk patients.
Diclofenac, 150 mg/d, appears to impair renal blood flow and glomerular
filtration rate. Elevation of serum aminotransferases occurs more commonly with
this drug than with other NSAIDs.
A
0.1% ophthalmic preparation is promoted for prevention of postoperative
ophthalmic inflammation and can be used after intraocular lens implantation and
strabismus surgery. A topical gel containing 3% diclofenac is effective for
solar keratoses. Diclofenac in rectal suppository form can be considered for
preemptive anal-gesia and postoperative nausea. In Europe, diclofenac is also
avail-able as an oral mouthwash and for intramuscular administration.
Diflunisal
Although diflunisal is
derived from salicylic acid, it is not metabo-lized to salicylic acid or
salicylate. It undergoes an enterohepatic cycle with reabsorption of its
glucuronide metabolite followed by cleavage of the glucuronide to again release
the active moiety. Diflunisal is subject to capacity-limited metabolism, with
serum half-lives at various dosages approximating that of salicylates (Table
36–1). In rheumatoid arthritis the recommended dose is 500–1000 mg daily in two
divided doses. It is claimed to be par-ticularly effective for cancer pain with
bone metastases and for pain control in dental (third molar) surgery. A 2%
diflunisal oral ointment is a clinically useful analgesic for painful oral
lesions.
Because its clearance
depends on renal function as well as hepatic metabolism, diflunisal’s dosage
should be limited in patients with significant renal impairment.
Etodolac
Etodolac is a racemic
acetic acid derivative with an intermediate half-life (Table 36–1). Etodolac
does not undergo chiral inversion in the body. The dosage of etodolac is
200–400 mg three to four times daily.
Flurbiprofen
Flurbiprofen is a
propionic acid derivative with a possibly more complex mechanism of action than
other NSAIDs. Its (S)(–) enantiomer
inhibits COX nonselectively, but it has been shown in rat tissue to also affect
tumor necrosis factor-α (TNF-α) and nitric oxide synthesis. Hepatic
metabolism is extensive; its (R)(+)
and (S)
(–) enantiomers are
metabolized differently, and it does not undergo chiral conversion. It does
demonstrate enterohepatic circulation.
Flurbiprofen is also
available in a topical ophthalmic formula-tion for inhibition of intraoperative
miosis. Flurbiprofen intrave-nously is effective for perioperative analgesia in
minor ear, neck, and nose surgery and in lozenge form for sore throat.Although
its adverse effect profile is similar to that of other NSAIDs in
most ways, flurbiprofen is also rarely associated with cogwheel rigidity,
ataxia, tremor, and myoclonus.
Ibuprofen
Ibuprofen is a simple
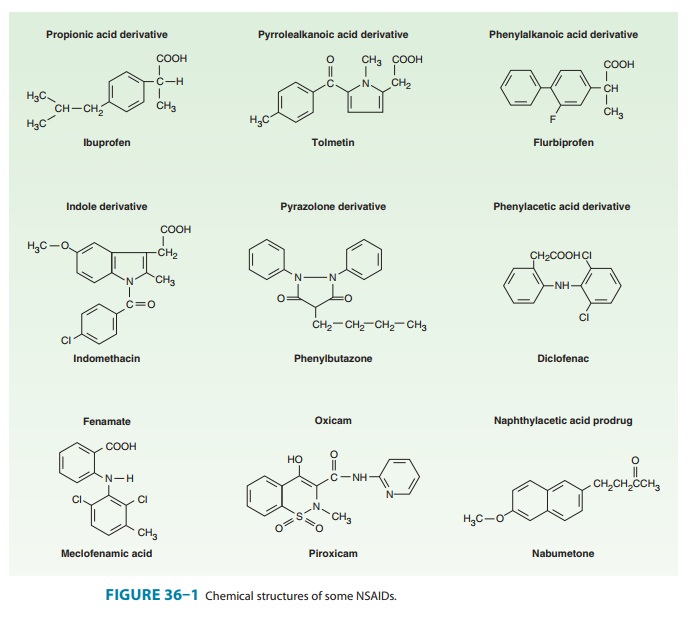
derivative of phenylpropionic acid (Figure 36–1). In doses of about 2400 mg
daily, ibuprofen is equivalent to 4 g of aspirin in anti-inflammatory effect.
Pharmacokinetic characteristics are given in Table 36–1.
Oral ibuprofen is
often prescribed in lower doses (<2400 mg/d), at which it has analgesic but
not anti-inflammatory efficacy. It is available over the counter in low-dose
forms under several trade names.
Ibuprofen is effective
in closing patent ductus arteriosus in preterm infants, with much the same efficacy
and safety as indo-methacin. The oral and intravenous routes are equally
effective for this indication. A topical cream preparation appears to be
absorbed into fascia and muscle; an (S
)(–) formulation has been tested. Ibuprofen cream was more effective than
placebo cream in the treatment of primary knee osteoarthritis. A liquid gel
preparation of ibuprofen, 400 mg, provides prompt relief and good overall
efficacy in postsurgical dental pain.
In comparison with
indomethacin, ibuprofen decreases urine output less and also causes less fluid
retention. The drug is rela-tively contraindicated in individuals with nasal
polyps, angio-edema, and bronchospastic reactivity to aspirin. Aseptic
meningitis (particularly in patients with systemic lupus erythematosus), and
fluid retention have been reported. Interaction with anticoagu-lants is
uncommon. The concomitant administration of ibuprofen and aspirin antagonizes
the irreversible platelet inhibition induced by aspirin. Thus, treatment with
ibuprofen in patients with increased cardiovascular risk may limit the
cardioprotective effects of aspirin. Furthermore, the use of ibuprofen
concomitantly with aspirin may decrease
the total anti-inflammatory effect.
Indomethacin
Indomethacin,
introduced in 1963, is an indole derivative (Figure 36–1). It is a potent
nonselective COX inhibitor and may also inhibit phospholipase A and C, reduce
neutrophil migration, and decrease T-cell and B-cell proliferation.
It differs somewhat
from other NSAIDs in its indications and toxicities.
It has been used to
accelerate closure of patent ductus arteriosus. Indomethacin has been tried in
numerous small or uncontrolled trials for many other conditions, including
Sweet’s syndrome, juve-nile rheumatoid arthritis, pleurisy, nephrotic syndrome,
diabetes insipidus, urticarial vasculitis, postepisiotomy pain, and
prophy-laxis of heterotopic ossification in arthroplasty.
An
ophthalmic preparation is efficacious for conjunctival inflammation and to
reduce pain after traumatic corneal abrasion. Gingival inflammation is reduced
after administration of indo-methacin oral rinse. Epidural injections produce a
degree of pain relief similar to that achieved with methylprednisolone in
post-laminectomy syndrome.
At
usual doses, indomethacin has the common side effects listed above. The GI
effects may include pancreatitis. Headache is expe-rienced by 15–25% of
patients and may be associated with dizzi-ness, confusion, and depression.
Rarely, psychosis with hallucinations has been reported. Renal papillary necrosis
has also been observed. A number of interactions with other drugs have been
reported . Probenecid prolongs indomethacin’s half-life by inhibiting both
renal and biliary clearance.
Ketoprofen
Ketoprofen is a
propionic acid derivative that inhibits both COX (nonselectively) and
lipoxygenase. Its pharmacokinetic character-istics are given in Table 36–1.
Concurrent administration of probenecid elevates ketoprofen levels and prolongs
its plasma half-life.The effectiveness of ketoprofen at dosages of 100–300 mg/d
is equivalent to that of other NSAIDs. In spite of its dual effect on
prostaglandins and leukotrienes, ketoprofen is not superior to other NSAIDs in
clinical efficacy. Its major adverse effects are on the GI tract and the
central nervous system (see common adverse effects above).
Ketorolac
Ketorolac
is an NSAID promoted for systemic use mainly as an anal-gesic, not as an
anti-inflammatory drug (although it has typical NSAID properties).
Pharmacokinetics are presented in Table 36–1. The drug is an effective
analgesic and has been used successfully to replace morphine in some situations
involving mild to moderate postsurgical pain. It is most often given
intramuscularly or intrave-nously, but an oral dose formulation is available.
When used with an opioid, it may decrease the opioid requirement by 25–50%. An
oph-thalmic preparation is available for ocular inflammatory conditions.
Toxicities are similar to those of other NSAIDs, although renal toxicity may be
more common with chronic use.x
Nabumetone
Nabumetone is the only
nonacid NSAID in current use; it is converted to the active acetic acid
derivative in the body. It is given as a ketone prodrug that resembles naproxen
in structure (Figure 36–1). Its half-life of more than 24 hours (Table 36–1)
permits once-daily dosing, and the drug does not appear to undergo
enterohepatic circulation. Renal impairment results in a doubling of its
half-life and a 30% increase in the area under the curve.Its properties are
very similar to those of other NSAIDs, though it may be less damaging to the
stomach than some other NSAIDs when given at a dosage of 1000 mg/d.
Unfortunately, higher dosages (eg, 1500–2000 mg/d) are often needed, and this
is a very expensive NSAID. Like naproxen, nabumetone has been associated with pseudoporphyria
and photosensitivity in some patients. Other adverse effects mirror those of
other NSAIDs.
Naproxen
Naproxen is a
naphthylpropionic acid derivative. It is the only NSAID presently marketed as a
single enantiomer. Naproxen’s free fraction is significantly higher in women
than in men, but half-life is similar in both sexes (Table 36–1). Naproxen is
effective for the usual rheumatologic indications and is available in a
slow-release formulation, as an oral suspension, and over the counter. A topical
preparation and an ophthalmic solution are also available.The incidence of
upper GI bleeding in over-the-counter use is low but still double that of
over-the-counter ibuprofen (perhaps due to a dose effect). Rare cases of
allergic pneumonitis, leukocy-toclastic vasculitis, and pseudoporphyria as well
as the common NSAID-associated adverse effects have been noted.
Oxaprozin
Oxaprozin is another
propionic acid derivative NSAID. As noted in Table 36–1, its major difference
from the other members of thissubgroup is a very long half-life (50–60 hours),
although oxapro-zin does not undergo enterohepatic circulation. It is mildly
urico-suric, making it potentially more useful in gout than some other NSAIDs.
Otherwise, the drug has the same benefits and risks that are associated with
other NSAIDs.
Piroxicam
Piroxicam, an oxicam
(Figure 36–1), is a nonselective COX inhib-itor that at high concentrations
also inhibits polymorphonuclear leukocyte migration, decreases oxygen radical
production, and inhibits lymphocyte function. Its long half-life (Table 36–1)
permits once-daily dosing.Piroxicam can be used for the usual rheumatic
indications. When piroxicam is used in dosages higher than 20 mg/d, an
increased incidence of peptic ulcer and bleeding is encountered. Epidemiologic
studies suggest that this risk is as much as 9.5 times higher with piroxicam
than with other NSAIDs (see common adverse effects above).
Sulindac
Sulindac
is a sulfoxide prodrug. It is reversibly metabolized to the active sulfide
metabolite, which is excreted in bile and then reabsorbed from the intestine.
The enterohepatic cycling prolongs the duration of action to 12–16 hours.In
addition to its rheumatic disease indications, sulindac suppresses familial
intestinal polyposis and it may inhibit the development of colon, breast, and
prostate cancer in humans. It appears to inhibit the occurrence of GI cancer in
rats. The latter effect may be caused by the sulfone rather than the sulfide.Among
the more severe adverse reactions, Stevens-Johnson epidermal necrolysis
syndrome, thrombocytopenia, agranulocyto-sis, and nephrotic syndrome have all
been observed. Like diclofenac, sulindac may have some propensity to cause
elevation of serum aminotransferases; it is also sometimes associated with
cholestatic liver damage, which disappears when the drug is stopped.
Tolmetin
Tolmetin is a
nonselective COX inhibitor with a short half-life (1–2 hours) and is not often
used. Its efficacy and toxicity profiles are similar to those of other NSAIDs
with the following excep-tions: it is ineffective (for unknown reasons) in the
treatment of gout, and it may cause (rarely) thrombocytopenic purpura.
Other NSAIDs
Azapropazone, carprofen, meclofenamate, and tenoxicam arerarely used and are not
reviewed here.
Related Topics