Changes Around Us | Term 2 Unit 3 | 7th Science - Student Activities | 7th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 7th Science : Term 2 Unit 3 : Changes Around Us
Student Activities
Look at the following list. Identify the physical and chemical changes and fill in the given table.
(rusting of iron, digestion of food, boiling egg, rotting banana, mixing sand and water, chopping wood, crushing a can, mixtures of different coloured buttons, burning of wood)

ACTIVITY 1
Melting of ice and freezing of water
Though ice and water look different, they are both made of water molecules. This means that no new substance is formed during the melting of ice, only a change of state from solid to liquid takes place during the melting of ice. So, the melting of ice to form water is a physical change.
The change which occurs during the melting of ice to form water can be reversed easily by freezing the water to form ice again by keeping a beaker of water in the freezer zone of a refrigerator.
ACTIVITY
Activity 2
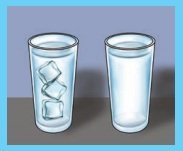
You must be remembering an activity done in Class six, in which we have taken two same shaped glasses and fill them with equal amount of water from same tap. We kept one under the hot sun and other under the shadow. After three to four hours, we saw that there is difference in water levels. The one kept in the hot place witness more evaporation compared to the one in shade. From this we can conclude that higher the temperature, the rate of evaporation will be more. As the temperature increases, more molecules are able to break free from the surface. Thus the rate of evaporation increases with rising temperature.
Activity 3
Take two pans, one wide and another narrow. Fill hot water in both to the same depth. Keep them in open. Observe after one to two hours. The pan that is wide has cooled more than the narrow one. That is more the surface area; the rate of evaporation is more.
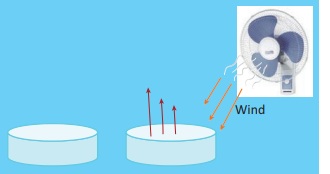
From this, can you guess why we unfurl the clothes while putting them to dry, rather than just drape them over the cloth line?
Greater the surface of conversion of a liquid, more molecules are available for evaporation.
Activity 4
Take sugar solution in a shallow, broad bowl. Place the bowl in hot sun for a few hours. See that the bowl does not get any disturbance for the whole day. You can see that the solvent in the sugar solution evaporates leaving the sugar crystals in the bowl.
ACTIVITY 5
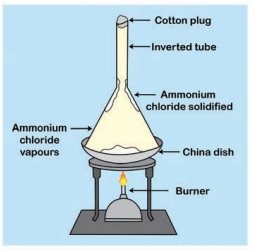
Sublimation
Take some camphor in a porcelain dish and cover it with a clean glass funnel. Close the mouth of the funnel with small amount of cotton wool. Heat the contents in the dish. can you see that camphor changes into vapour state without becoming liquid.
Ammonium chloride is another substance that undergoes sublimation.
ACTIVITY 6
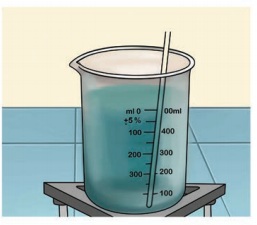
Crystallizing copper sulphate
Take about 100ml of water in a beaker. Heat the water over a burner till it boils. Add impure copper sulphate to the hot water with constant stirring. Continue to add copper sulphate till the solution takes up the added copper sulphate, that is, the added copper sulphate will not dissolve anymore. Filter the contents on a glass plate and allow it to cool. You could see crystals of copper sulphate in a few hours.
When food gets spoiled, it produces a foul smell. Shall we call this change as a chemical change?
Yes, it is a chemical change.
Discuss in the class. Give your reflections.
Try yourself
Cut a fresh slice of potato and brinjal and keep it away for sometime.

Discuss and give your answer
You know that plant produce their food by a process called photosynthesis.
Can we call photosynthesis a chemical change? Yes
The Iron Pillar at Delhi
Amazingly there is an iron that did not rust! There is an iron pillar at the Qutub complex in Delhi which is more than 1600 years age. Even after such a long period, the iron pillar kept in open spaces has not rusted at all. This shows that Indian scientists made great advances in metal making technology even at 16th century which enabled them to make this iron pillar having the quality of great rust resistance.

Another way of preventing rusting is to deposit a layer of a metal like chromium or zinc on iron. This is called galvanization and you will learn about this detail in higher classes.

ACTIVITY 7
Take a small piece of magnesium ribbon and clean it by rubbing its surface with a sand paper. Hold the magnesium ribbon at one end with a pair of tongs and bring its other end over the flame of a burner.
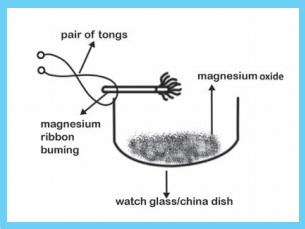
Magnesium ribbon must be cleaned before burning. So that the layer of magnesium oxide can be removed in order to get the desired chemical reaction.
Magnesium ribbon burns in air with a dazzling flame and forms a white ash, I magnesium gets oxidised to magnesium oxide.
ACTIVITY 8
When baking soda and lemon juice are mixed together, then bubbles of carbon-di-oxide are formed along with the formation of some salt and water. Take 10 ml of lemon juice and add pinch by pinch of baking soda to it. Actually when we mix baking soda with lemon juice, we will hear a hissing sound when bubbles of carbon-di-oxide coming out and rising in the reaction vessel.
ACTIVITY 9
Ask a student to stretch both hands, put a pinch of soap powder in one hand and a pinch of glucose in the other hand. Add a few drops of water to soap powder and ask how the student feels upon adding water. Now add a few drops of water to the glucose at the other hand. Now ask the student how he /she feels on adding water, What is the feeling when water is added to glucose?
What is the difference when water is added to soap powder and when water is added to glucose?
Every year we observe that seasons changes. We go from rains to winter and winter to summer and so on.
* What types of clothes are worn in winter?
* What are the clothes that we wear in summer?
Related Topics