Term 2 Unit 3 | 7th Science - Changes Around Us | 7th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 7th Science : Term 2 Unit 3 : Changes Around Us
Changes Around Us
Unit 3
Changes Around Us

Learning Objectives
* To state the effect of heat on solid, liquid and gas and the associated changes in the arrangement of particles upon heating
* To differentiate physical change and chemical change on the basis of particle theory
* To involve in experiments crystallizing copper sulphate, melting ice, freezing water, sublimating camphor.
* To identify the process as a physical change or chemical change based on its characteristics
* To clarify the process of rusting, burning of paper, curdling of milk, reaction of baking soda with lemon juice
* To distinguish periodic and non-periodic changes
* To experience the endothermic and exothermic changes through simple activities
Introduction
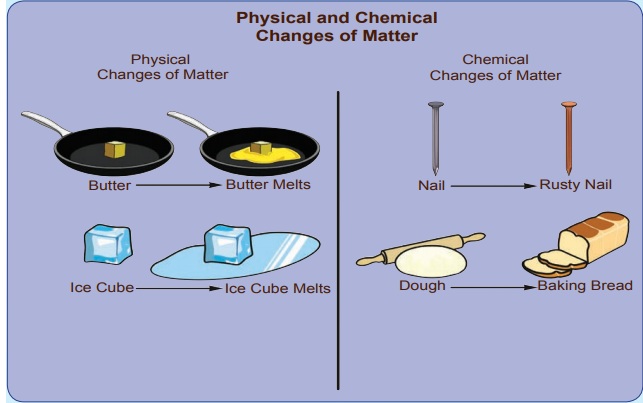
Changes take place around us all the time. A change refers to an alteration in physical properties or alteration in the composition of matter. For example, ice melts on heating, that is, it changes from a solid to liquid. On further heating, water starts evaporating; it changes from a liquid to gas. Here, there is a change in the physical state of the substance. Let us look at another change, that is, when objects made of iron are exposed to moist conditions, a reddish-brown new substance called rust forms on the surface of these objects. In this instance of rusting, there is change in the composition of the substance. Thus, the change involves an alteration in the properties such as colour, texture and the state of the substance since there is formation of a new substance.
Let us go for another set of example. Heat a cup of water and a paper. The water upon heating become just hotter and hotter and at some point will become water vapour. It remains water at all times; that is, water remains the same, only its volume changes and hence it is called as physical change. Whereas in case of burning of paper, changes to carbon dioxide and other substances. Now we cannot get back the paper after burning. As there is a change in the chemical nature, it is called as chemical change.
When you mix sugar in water, is it a chemical change or physical change?
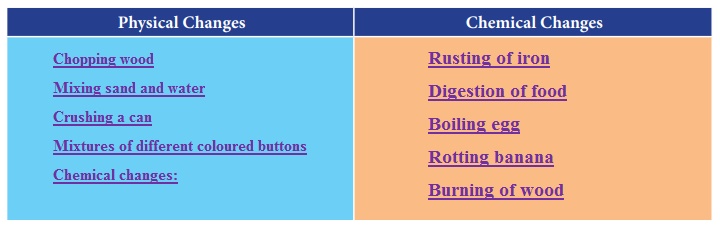
Look at the following list. Identify the physical and chemical changes and fill in the given table.
(rusting of iron, digestion of food, boiling egg, rotting banana, mixing sand and water, chopping wood, crushing a can, mixtures of different coloured buttons, burning of wood)

In class six, we read that matter is classified as solid, liquid and gas based on the physical state. We know that matter is made up of tiny particles, atoms and molecules; particles are in constant and random movement. Let us have a look at the summary of the characteristics of solid, liquid and gas.
When the arrangement of the particles in a substance change for any reason (applying pressure, altering temperature and other different reasons) the physical state of the substance gets changed.
Let us see what happens when we apply heat to the substances.
Related Topics