Changes Around Us | Term 2 Unit 3 | 7th Science - Exothermic and Endothermic chemical changes | 7th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 7th Science : Term 2 Unit 3 : Changes Around Us
Exothermic and Endothermic chemical changes
Exothermic
and Endothermic
chemical changes
Just as the physical change, Chemical reaction will
be either endothermic or exothermic.
ACTIVITY 9
Ask a student to stretch both hands, put a pinch of soap powder in one hand and a pinch of glucose in the other hand. Add a few drops of water to soap powder and ask how the student feels upon adding water. Now add a few drops of water to the glucose at the other hand. Now ask the student how he /she feels on adding water, What is the feeling when water is added to glucose?
What is the difference when water is added to soap powder and when water is added to glucose?
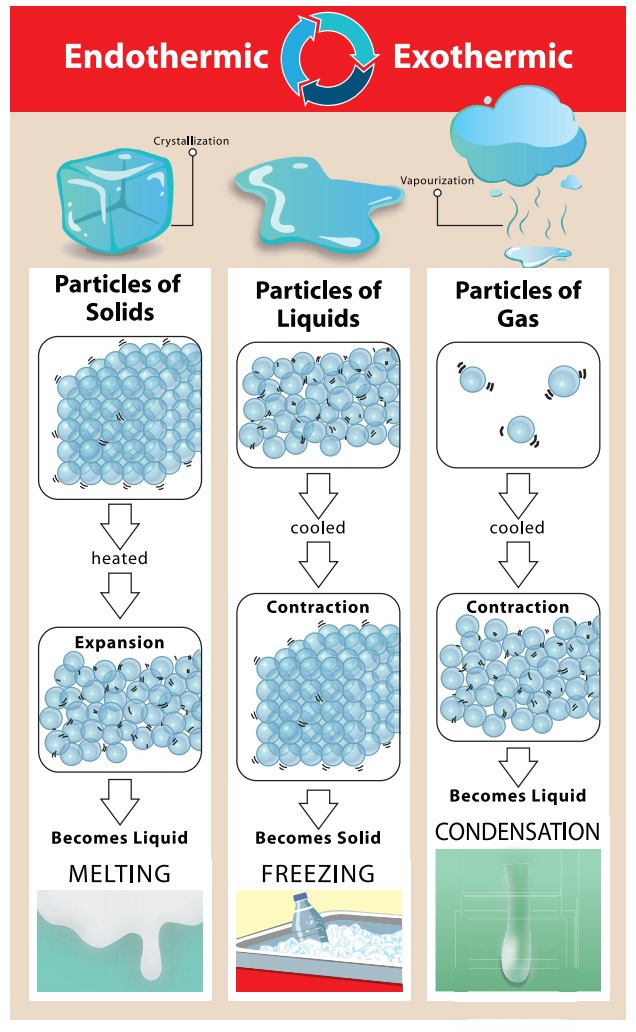
In this activity, the student reported that he / she felt the warmness in the palm when water is added to soap powder. Right! We saw that the burning of magnesium ribbon gives out heat and light. Similarly, burning of wood also releases heat and light. Such changes in which heat is released are known as exothermic changes.
There are some changes in which heat is absorbed.
For example, water absorbs heat when it evaporates to form water vapours.
Similarly ice absorbs heat when it melts to form water. Such changes in which
heat is absorbed are known as endothermic changes. Dissolution of glucose in
water is also an endothermic change.
Related Topics