Changes Around Us | Term 2 Unit 3 | 7th Science - Questions Answers | 7th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 7th Science : Term 2 Unit 3 : Changes Around Us
Questions Answers
Evaluation
I. Choose
the best answer
1. When a
woollen yarn is knitted to get a sweater, the change
can be classified as _______
.
a. physical change
b. chemical change
c. endothermic change
d. exothermic change
Answer: 1. Answer: a. physical change
2. ___________
of the following are endothermic changes.
a. Condensation and melting
b. Condensation and freezing
c. Evaporation and melting
d. Evaporation and freezing
Answer: c. Evaporation and melting
3. The
chemical change is ____________ .
a. water to clouds
b. growth of a tree
c. cow dung to bio-gas.
d. ice-cream to molten ice-cream.
Answer: c. cow dung to bio-gas
4. ________is
an example of a periodic change.
a. Earthquake.
b. Formation of rainbow in sky
c. Occurrence of tides in seas.
d. Showering of rain
Answer: c. Occurrence of tides in seas.
5. _________
is not a chemical change.
a. Dissolution of ammonia in water
b. Dissolution of carbon-di-oxide in water
c. Dissolution of oxygen in water
d. Melting of polar ice caps
Answer: d. Melting of polar ice caps
II. Fill
in the blanks
1. Filling up a balloon with hot air is a physical change.
2. Stretching gold coin into a ring is a physical change.
3. Opening a gas cylinder knob converts liquid fuel
into gas fuel. This is an example of physical change.
4. Spoiling of food is a chemical change.
5. Respiration is a periodic change.
III. True
or False. If false, give the correct answer.
1. Cutting
of cloth is an example of a periodic change.
Cutting of cloth is an example of a physical change.
2. Taking a glass of water and freezing it by
placing it in the freezer is a chemical change.
Taking a glass of water and
freezing it by placing it in the freezer is a physical change.
3. A bean plant collecting sunlight and turning it
into bean seeds is an example of physical and non-periodic change.
A bean plant collecting sunlight and turning it into bean seeds is an example of chemical and periodic change.
4. If the chemical properties of a substance
remain unchanged and the appearance or shape of a substance changes it is
called a periodic change.
If the chemical properties of a substance remain unchanged and the appearance or shape of a substance changes it is called a physical change.
5. Tarnishing of silver is an example of
endothermic change.
Tarnishing of silver is an example of chemical change.
IV. Match
the following
A B C
1. Melting :
Change of state from liquid to solid -
Ticking of clock
2. Condensation : Change
of state from liquid to gas - Formation of ice cube
3. Evaporation : Change of state from solid to liquid -
Collecting flowers
4. Freezing :
Change of state from gas to liquid
- Ice cube to water
5. Periodic change : Occurs
at irregular time intervals - Water to steam
6. Non-periodic change : Occurs at regular time intervals -
Steam to water drops
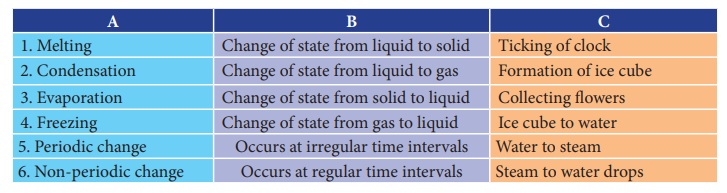
Answer
1. Melting : Change of state from solid to
liquid - Ice cube to water
2. Condensation : Change of state from gas to
liquid - Steam to water drops
3. Evaporation : Change of state from liquid to
gas - Water to steam
4. Freezing : Change of state from liquid to
solid - Formation of ice cube
5. Periodic change
: Occurs at
regular time intervals - Ticking of clock
6. Non-periodic
change : Occurs at irregular time intervals - Collecting flowers
V. Classify
the following changes as physical and chemical changes
A rough piece of wood is sanded and polished
resulting in change in texture, Rusting of a iron nail, Painting the grill,
Bending a paper clip, Pounding silver into thin plate, Rolling the chappathi dough into thin wire, Occurrence of
day and night, eruption of volcano, burning of matchstick, dosa from the
batter, blinking of eyelids, occurrence of a thunderstorm, rotation of the
earth, formation of eclipses.
Physical changes
1. A rough piece of wood is sanded and polished resulting in
change in texture
2. Painting the grill
3. Bending a paper clip
4. Pounding silver into thin plate
5. Rolling the chappathi dough into thin layer
6. Blinking of eyelids
Chemical changes
1. Rusting of an iron nail
2. burning of matchstick
3. dosa from the batter
Periodic change
1. Occurrence of day and night
2. Rotation of the earth
3. Formation of eclipses.
Non-periodic change
1. eruption of volcano
2. occurrence of a thunderstorm
VI. Analogy
1. Physical Change: Boiling::Chemical Change: Rusting of iron.
2. Wood to saw dust: Physical change :: Wood to Ash:
Chemical change
3. Forest fire: Non-periodic change change::Change in period in
a school: periodic change
VII. Very
short answer type question
1. State two examples of periodic changes.
(i) Rotation and Revolution of the earth
(ii) Beating of the heart
2. Mention any two exothermic reactions.
(i) Water freezes into ice by giving out heat.
(iii) Steam condenses into water by giving out heat.
3. Cold
milk is heated and it becomes hot. Which type of change it is?
Cold milk when heated
becomes hot. It is a physical change.
4. What
type of change is artificial ripening of fruit?
Artificial ripening of
fruit involves the use of chemicals. It is a chemical change.
5. What
type of change is colouring of a paper?
Colouring of paper is a
physical change.
6. Growing
of nails is a periodic change. Why?
Nails keep growing little
by little every day. The growth is repeated as long as one is alive. So the
growing of nails is a periodic change.
7. What
type of energy changes is associated when ice melts?
Ice absorbs heat and
becomes water. It is an endothermic energy change. Melting of ice involves
change in state. Solid state is changed into liquid state. It is a physical
change.
VIII. Short
answer type question
1. Distinguish
physical and chemical changes.
Physical changes are the changes in which only physical
properties of a substance undergo a change and there is no change in its
chemical composition.
Changes that occur with the formation of new substance with
different chemical composition are called chemical changes. They are associated
with the evolution or absorption of heat or light energy.
2. How
can a change occur in a substance?
A change may occur in colour, state and texture of a substance.
Physical properties such as lustre, malleability and ductility, density,
viscosity, solubility, mass, volume undergo some change.
3. Can
you suggest a method to collect water from sea water?
Sea water can be boiled and the steam that comes out must be
cooled. When steam condenses it becomes water. This water is pure and it is
free from salts. This process is known as distillation.
4. Is
solar eclipse a periodic change? Give your reason.
The earth and the moon revolve round the sun. When the moon
comes in between the sun and the earth, the earth is engulfed in the shadow of
the moon. This is called solar eclipse. It occurs at regular intervals. So it
is called a periodic change.
5. What
is the difference between dissolution of sugar and burning of sugar?
(i) Dissolution of sugar is a physical change. The sugar
dissolved in water sets deposited under the water after some time. This is only
a temporary change.
(ii) When sugar is burnt it undergoes chemical change and only
black carbon remains as residue. We cannot get back sugar from it. So it is
called a chemical change.
IX. Long
answer type question
1. Explain
the following statement: Digestion is a chemical change.
Digestion is a chemical change. The food we eat is chewed in the
mouth. The enzymes in the saliva bring about some changes in the food. Then it
goes to the stomach. It is broken down into smaller and smaller components. A
number of enzymes play important roles in digesting carbohydrates, proteins and
lipids. The smaller substances are absorbed by small intestines. Then they join
the blood stream. Thus during digestion, many changes take place in the food we
eat. From the enzymes in saliva to the enzymes in the stomach many enzymes act
on the food and bring about chemical changes. These changes are permanent and
irreversible. So digestion is called a chemical change.
2. How
the iron blade is fixed into a wooden handle in tools used to dig the soil?
It is not easy to fix a wooden handle into hole in the iron
blade. To fix it, they heat the hole of the iron blade. When it is heated
sufficiently the hole in the iron blade expands. Now one end of the wooden
handle is inserted into the hole easily. Then cool water is sprayed on the iron
blade. On cooling the hole contracts and it grips the wooden handle tightly.
This kind of heating and cooling brings about a physical change.
X. Higher
order Thinking questions
1. Peeled
and unpeeled banana does not look the same. Does that mean peeling banana is a
chemical change?
A peeled banana is exposed to air. It reacts with the gases in
the air and changes colour. So the peeled banana undergoes a chemical change.
As the unpeeled banana is protected by a skin it doesn't undergo any change.
2.
A very hot glass on putting in cold water cracks. What does this change
indicate?
A hot glass is in an
expanded state. When cold water is put in it the hot glass shrinks suddenly. As
a result it cracks. This is a physical change brought about by heat and
coldness. The glass may crack but it does not undergo any chemical change.
3.
Boiling of water is a physical change; but boiling of egg is a chemical change.
Why?
The boiled water becomes steam. When the steam is cooled, we get
water once again. This is a change of state and it is a physical change.
But when egg is boiled, the contents of the egg undergo
permanent, irreversible chemical changes. We cannot bring the egg to its former
position. So it is called a chemical change.
XI. Assertion
– Reason type question
1. Assertion:
The explosion of fire cracker is a physical change.
Reason: A
physical change is
a reversible change
a. Both A and R are true and R is the correct
explanation of A.
b.
Both A and
R are true
but R is not
the
correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Answer : d. A is false
but R is true.
2. Assertion: The
process of conversion
of liquid water
to its vapours
by heating the
liquid is called boiling.
Reason:
The process of conversion of water vapours
to liquid by
cooling the vapours
is called condensation.
a. Both A and R are true and R is the correct
explanation of A.
b.
Both A and
R are true
but R is
not the correct explanation of A.
c. A is true but R is false
d. A is false but R is true.
Answer : b. Both A and
R are true and R is not the correct explanation of A.
3.
Assertion: Burning of wood log to charcoal is a physical change.
Reason: The
products formed of
burning a piece of wood can be easily converted back to
wood log.
a. Both A and R are true and R is the correct
explanation of A.
b.
Both A and
R are true
but R is
not the correct explanation of A
c. A is true but R is false
d. A is false but R is true.
Answer : Note i) Burning of wood into charcoal is a chemical change.
ii)
The charcoal cannot be converted into a wooden log. So both Assertion and
Reason are false.
4. Assertion:
The formation of iron oxide from iron is a chemical change.
Reason: For
the rust to
form from iron,
it must be exposed to air and
water.
a. Both A and R are true and R is the correct
explanation of A.
b.
Both A and
R are true
but R is
not the correct explanation ofA.
c. A is true but R is false.
d. A is false but R is true.
Answer : a. Both A and
R are true and R is the correct explanation of A.
5.
Assertion: A drop
of petrol when
touched with finger gives a chill
feeling. Reason: The
above phenomenon is
an endothermic one.
a. Both A and R are true and R is the correct
explanation of A.
b.
Both A and
R are true
but R is not the
correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Answer : a. Both A and
R are true and R is the correct explanation of A.
XII. Picture based question
1.
Observe the picture and list down the changes that are accompanied in the
picture.

a) Solid wax becomes liquid.
b) The liquid turns into gas and bums.
c) The molten liquid wax condenses into solid wax at the bottom
2. Observe
the picture containing a kettle and note that it has salt water in it and
answer the following questions:
a. What
is name of the process that is done to the kettle?
Boiling
b. What
will happen to the content of the kettle?
Water in the kettle becomes
steam.
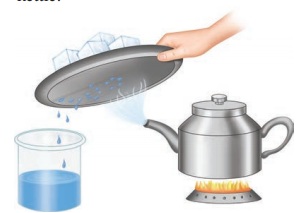
c. What
kind of change is occurring on the cold surface of the metal plate?
A physical change called
condensation.
d. What
can you say about the quality of water that is obtained in the beaker?
The water obtained in the
beaker is pure. It is free from all kinds of salts.
Related Topics