Changes Around Us | Term 2 Unit 3 | 7th Science - Chemical changes | 7th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 7th Science : Term 2 Unit 3 : Changes Around Us
Chemical changes
Chemical changes
Changes that occur with the formation of new substance with different chemical composition or transformation of a substance into another substance with the evolution or absorption of heat or light energy are termed as chemical changes. Rusting of iron, burning, curdling of milk, reaction of baking soda with lemon juice, fermentation are some examples of chemical changes.
Chemical changes are very important in our lives. All the new substances which we use in various fields of our life are produced as a result of chemical reactions. Some of the examples of the importance of chemical changes are given below:
i. Metals are extracted from their naturally occurring compounds called ‘ores’ by a series of chemical changes.
ii. Medicines are prepared by carrying out a chain of chemical changes.
iii. The materials such as plastics, soaps, detergents, perfumes, acids, bases, salts etc are all made by carrying out various types of chemical changes.
iv. Every new material is discovered by studying different types of chemical changes.
In addition to new products, the following may also accompany a chemical change:
* Heat, light or any other radiation may be given off or absorbed.
* Sound may be produced
* A change in smell may take place (or) a new smell may be given off.
* A colour change may take place.
* A gas may be formed.
Explosion of a firework is a chemical change. We know that such an explosion produces heat, light, sound and unpleasant gases that pollute the atmosphere. That is why we are advised not to play with fireworks.
When food gets spoiled, it produces a foul smell. Shall we call this change as a chemical change?
Yes, it is a chemical change.
Discuss in the class. Give your reflections.
You must have noticed that a slice of an apple acquires a brown colour if it is not consumed immediately. Colour of the potato remains the same when stored in water but there is change in colour with the piece kept in air. Look at the cut brinjal kept in air. The change of colour in these cases is due to the formation of some new substances which you will learn in higher classes. Are these not chemical changes?
Try yourself
Cut a fresh slice of potato and brinjal and keep it away for sometime.

Discuss and give your answer
You know that plant produce their food by a process called photosynthesis.
Can we call photosynthesis a chemical change? Yes
1. Rusting of iron
In class six, we have already studied that rusting is an example of a chemical change. Now, shall we read why the process of rusting is called a chemical change.
The Iron Pillar at Delhi
Amazingly there is an iron that did not rust! There is an iron pillar at the Qutub complex in Delhi which is more than 1600 years age. Even after such a long period, the iron pillar kept in open spaces has not rusted at all. This shows that Indian scientists made great advances in metal making technology even at 16th century which enabled them to make this iron pillar having the quality of great rust resistance.

Rusting is one change that affects iron articles and slowly destroys them. Since iron is used in making bridges, ships, cars, truck bodies and many other articles, the monetary loss due to rusting is huge. The process of forming rust is represented as follows:
iron + oxygen + water → rust
2Fe + 2O2 from air + 2H2O → 2Fe2O3 . H2O
For rusting to take place both oxygen and water (or even water vapour) is essential. In fact, if the content of moisture in air is high, the air is said to be more humid and eventually rusting is faster.
How can we prevent rusting?
Iron articles can be prevented from making contact with oxygen, water/water vapour. A simple way is to apply a coat of paint or grease. These coats should be applied regularly to prevent rusting.
Another way of preventing rusting is to deposit a layer of a metal like chromium or zinc on iron. This is called galvanization and you will learn about this detail in higher classes.

2. Burning
We have already studied that burning of paper is a fast change. Burning a piece of paper gives entirely new substances such as carbon-di-oxide, water, water vapour, smoke and ash. Heat and light are also given out during the burning of paper.
ACTIVITY 7
Take a small piece of magnesium ribbon and clean it by rubbing its surface with a sand paper. Hold the magnesium ribbon at one end with a pair of tongs and bring its other end over the flame of a burner.
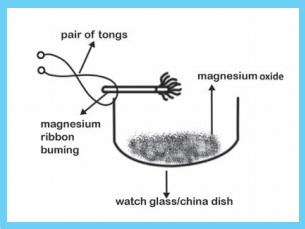
Magnesium ribbon
must be cleaned before burning. So that the layer of magnesium oxide can be
removed in order to get the desired chemical reaction.
Magnesium ribbon
burns in air with a dazzling flame and forms a white ash, I magnesium gets
oxidised to magnesium oxide.
We cannot combine the products of burning of paper to form the original paper again. So, it is a permanent change. Now, shall we perform an activity of burning a piece of magnesium ribbon and find what type of change is it?
What do you observe?
You can see that the magnesium ribbon starts burning with a dazzling white light. Hold the burning magnesium ribbon over a watch glass so that the powdery ash being formed by the burning of magnesium collects in the watch glass.
When magnesium ribbon burns in air, then the magnesium metal combines with the oxygen of air to form a new substance called magnesium oxide.
Magnesium + Oxygen → Magnesium oxide
2Mg + O2 → 2MgO
Magnesium oxide compound appears as a white powdery ash.
The burning of magnesium ribbon is a chemical change, because a new substance, magnesium oxide, is formed during this change.
3. Curdling of milk
We know that curdling of milk is an example of irreversible change since we cannot get back the milk after curdling occurs. It is also called as a chemical change. Shall we clarify the process of curdling?
Curdling is a process in which liquid gradually turns into solid, forming clumps along the way. Take hot milk in a pan and add few drops of curd, in few minutes milk curdles forming lumpy solid masses. We can even add lemon extract to the hot milk to effect curdling immediately, but the taste and texture of the curd will not be the same as that of the curdling occurring in a few hours. You can try to taste the curd formed by immediate curdling and gradual curdling.
4. Fermentation
In class six, we saw an example that preparation of batter to produce idly is an example for irreversible change.
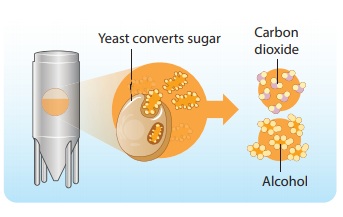
Fermentation is the process in which microorganisms such as yeast and certain bacteria break down sugar solution into alcohol and carbon-di-oxide.
It is an irreversible process as the alcohol formed cannot be turned back into sugar. Thus, fermentation is a chemical change.
Louis Pasteur (1822-1895), a French chemist and microbiologist was the first person to describe the process of fermentation.
He described that fermentation occurs in the absence of air and in the presence of micro organisms such as yeast. He discovered the cure for rabies.

5. Chemical reaction of baking soda with lemon
Baking soda is sodium hydrogen carbonate and lemon juice contains citric acid. So, when these two substances are mixed together, then a chemical change takes place between sodium hydrogen carbonate and citric acid to form three new substances: sodium citrate, carbon-di-oxide and water. The chemical change can be written in the form of a word equation as follows:-
Sodium hydrogen carbonate + citric acid → sodium citrate + carbon dioxide + water
ACTIVITY 8
When baking soda and lemon juice are mixed together, then bubbles of carbon-di-oxide are formed along with the formation of some salt and water. Take 10 ml of lemon juice and add pinch by pinch of baking soda to it. Actually when we mix baking soda with lemon juice, we will hear a hissing sound when bubbles of carbon-di-oxide coming out and rising in the reaction vessel.
Related Topics