Changes Around Us | Term 2 Unit 3 | 7th Science - Effect of heat on solid, liquid and gases | 7th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 7th Science : Term 2 Unit 3 : Changes Around Us
Effect of heat on solid, liquid and gases
Effect of heat
on solid, liquid and gases
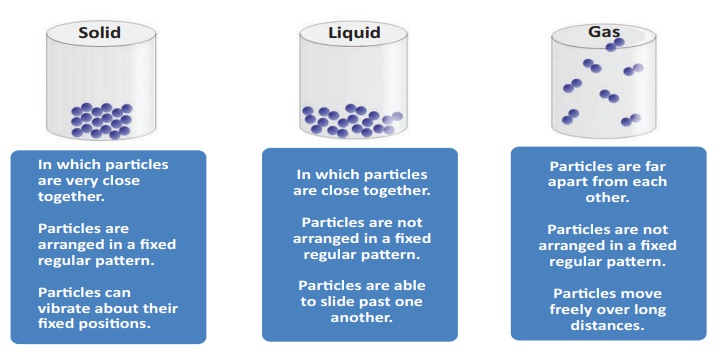
Upon heating, particle arrangement within the
state of matter gets disturbed. The disturbance is seen either as expansion or
contraction.
When heated or cooled, the object may expand or
contract, but the mass remains the same. That is, the number of particles that
was inside the object does not undergo any change, only the arrangement of the
particle changes. When a glass of water is heated, its volume increases and if
a glass of water is cooled its volume decreases.
Such changes where there is change in volume but
mass remaining the same are called physical changes and they can be pictorially
depicted as follows:

There are other possibilities that can occur upon
heating the solids, liquids and gases. The possible changes are due to melting,
boiling, freezing and condensation during which there is change in the physical
state of the particles of the matter. Let us discuss about them in detail in a
short while.
Let us now see some physical changes and the
underlying reasons as why they are simply physical changes.
Related Topics