Chapter: Medical Immunology: The Induction of an Immune Response: Antigens, Lymphocytes, and Accessory Cells
Stimulation of a B-Lymphocyte Response by a T-Dependent Antigen
Stimulation of a B-Lymphocyte Response by a T-Dependent Antigen
In contrast to T lymphocytes, B lymphocytes recognize external epitopes of unprocessed antigens, which do not have to be associated to MHC molecules. Some special types of APC, such as the Langerhans cells of the epidermis and the follicular dendritic cells of the germinal centers, appear to adsorb complex antigens onto their membranes, where they are expressed and presented for long periods of time. Accessory cells and helper T lympho-cytes provide additional signals necessary for B-cell activation, proliferation, and differen-tiation. A major role is believed to be played by a complex of four proteins associated non-covalently with the membrane immunoglobulin, including CD19 and CD21. These proteins appear to potentiate the signal delivered through occupancy of the binding site on membrane immunoglobulin.
Similar to the TcR, membrane immunoglobulins have short intracytoplasmic do-mains that do not appear to be involved in signal transmission. At least two heterodimers composed of two different polypeptide chains, termed Igα and Igβ , with long intracyto-plasmic segments are associated to each membrane immunoglobulin. These heterodimers seem to have a dual function:
1. They act as transport proteins, capturing nascent immunoglobulin molecules in the endoplasmic reticulum and transporting them to the cell membrane.
2. They are believed to be the “docking sites” for a family of protein kinases related to the src gene product, including p56lck and p59fyn, that also play a role in T-cell activation. In addition, the phosphatase CD45 is essential for p56lck activation, thus initiating a cascade of tyrosine kinase activation. Specific to B-cell activa-tion is the involvement of a specific protein kinase known as Bruton’s tyrosine kinase (Btk) in the kinase cascade. The critical role of this kinase was revealed when its deficiency was found to be the cause of infantile agammaglobulinemia (Bruton’s disease).
The subsequent sequence of events seems to have remarkable similarities to the acti-vation cascade of T lymphocytes. Activation and translocation of common transcription factors (e.g., NF-AT, NF- B) induce overlapping but distinct genetic programs. For in-stance, in B cells NF- B activates the expression of genes coding for immunoglobulin polypeptide chains. For a B cell to complete its proliferation and differentiation into anti-body-producing plasma cell or memory B cell, a variety of additional signals are required.
In the case of the stimulation of a B-cell response with a T-dependent antigen, the ad-ditional signals are delivered by helper T cells in the form of both soluble factors (cy-tokines) and interactions between complementary ligands (costimulatory molecules) ex-pressed by T cells and B cells. A naive B cell is initially stimulated by recognition of an epitope of the immunogen through the membrane immunoglobulin. Two other sets of membrane molecules are involved in this initial activation-the CD45 molecule and the CD19/CD21/CD81 complex. Whether the activation mediated by CD45 involves interac-tion with a specific ligand on the accessory cell remains to be determined. In the CD19/CD21/CD81 complex the only protein with a known ligand is CD21, a receptor for C3d (a fragment of the complement component 3, C3). It is possible that B cells interact-ing with bacteria coated with C3 and C3 fragments may receive a costimulatory signal through the CD19/CD21/CD81 complex.
In the same microenvironment where B lymphocytes are being activated, helper T lymphocytes are also activated. Two possible mechanisms could account for this simulta-neous activation:
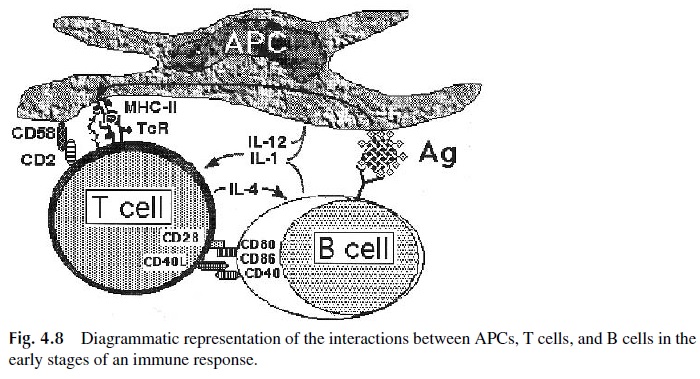
· The same accessory cell (i.e., a macrophage) may present not only membrane-ab-sorbed, unprocessed molecules with epitopes reflective of the native configu-ration of the immunogenic molecule to B lymphocytes, but also MHC II–asso-ciated peptides derived from processed antigen to the helper T lymphocytes (Fig. 4.8).
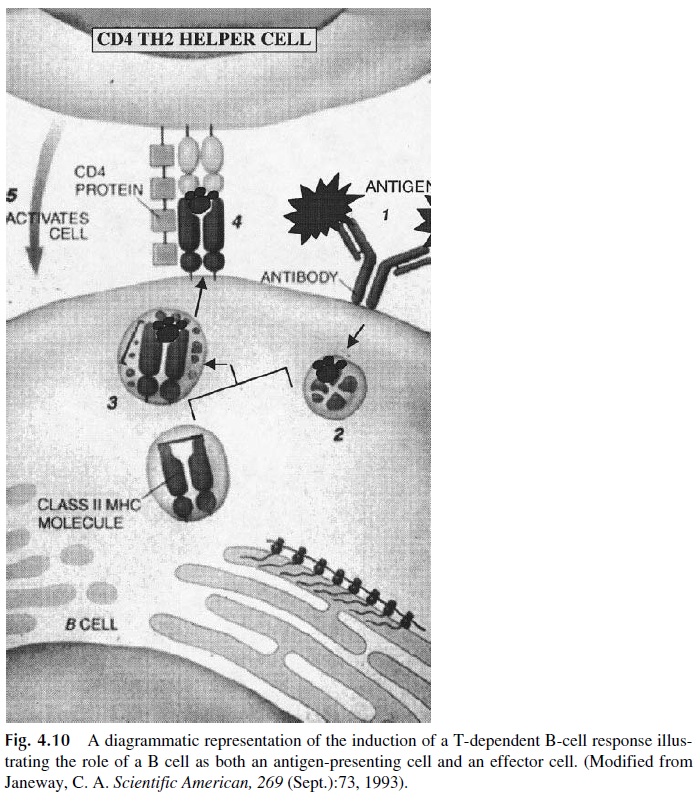
· The B cell may internalize the immunoglobulin-antigen complex, process the anti-gen, and present MHC II–associated peptides to the helper T cells (Fig. 4.10).
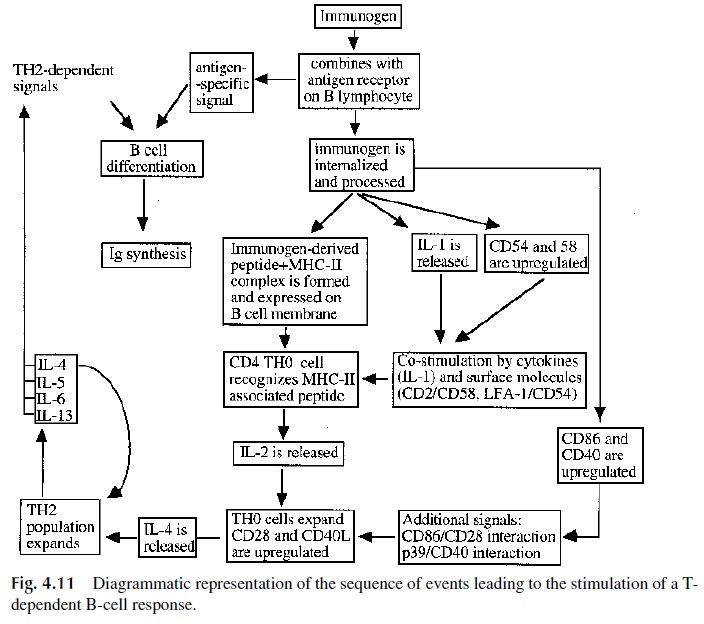
The proper progression of the immune response will require complex interactions be-tween accessory cells (macrophages or B cells), helper T lymphocytes, and B lymphocytes (Fig. 4.11). The helper T cell, as discussed earlier, receives a variety of costimulatory sig-nals from APCs, and the activated helper T cell, in turn, delivers activating signals to APC and B cells. Some of the signals are mediated by interleukins and cytokines, such as IL-2 and IL-4, that stimulate B-cell proliferation and differentiation, and interferon-γ, which in-creases the efficiency of APC, particularly macrophages. Other signals are mediated by cell-cell interactions involving CD40L (on T cells) and CD40 (on B cells). As a conse-quence of signaling through the CD40 molecule, B cells express CD80 and CD86, which deliver differentiation signals to T cells through the CD28 family of molecules. Additional activation signals are then delivered.
The differentiation of helper T lymphocytes results in specialized functions to assist B-cell activation and differentiation (TH 2 lymphocytes) and to assist the proliferation and differentiation of cytotoxic T lymphocytes and natural killer (NK) cells (TH1 lymphocytes). These two subpopulations cannot be defined on the basis of expression of any specific membrane markers.
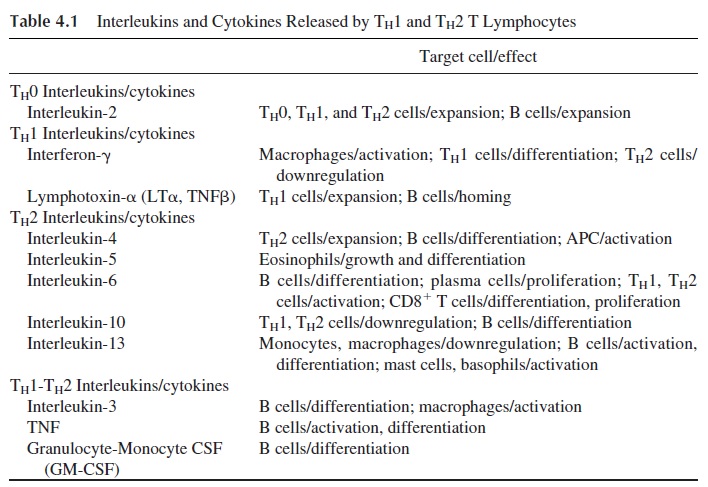
Their definition is based on the repertoire of cytokines they release: TH1 cells predominantly release IFNγ and TNFβ , and TH 2 cells predominantly release IL-4 and IL-10 (Table 4.1).
Several factors appear to control the differentiation of TH1 and TH 2. The early stages of proliferation of TH0 cells (as the common precursors are designated) are IL-2 dependent. IL-2 is the main cytokine releases by activated TH0 cells and has both autocrine and paracrine effects, thus promoting TH0 proliferation. As the cells continue to proliferate, IL-12 promotes TH1 differentiation while IL-4 promotes TH 2 differentiation. The cellular source of IL-12 is the APCs, and experimental work suggests that specific antigens or bac-terial products with adjuvant properties may induce the release of IL-12 by those cells, thus tilting the immune response towards TH1 differentiation. The cellular source of the IL-4 needed to initiate the differentiation of TH 2 cells is not clear. Recent research suggests that the initial drive towards TH 2 differentiation may be provided by at least two specialized cell populations.
One is a special population of CD4+ T cells expressing a marker known as NK1.1, first defined in IL-2–activated NK cells. These T cells carry a high quantity of pre-formed IL-4 in their cytoplasm, ready to be released after proper stimulation. What factors control the activation of this specialized population are not known, but once IL-4 is released and TH 2 cells start to differentiate, these cells continue to release IL-4 and promote further TH 2 differentiation. The other is a subpopulation of dendritic cells known as DC2, which seems to promote TH 2 differentiation independently of IL-4 synthesis. The other DC pop-ulation, DC1, secretes IL-12 and interferon-α and promotes the differentiation of TH1 cells.
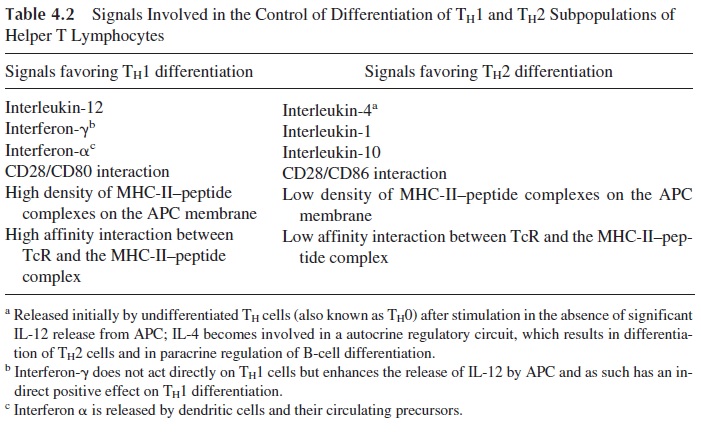
Several additional factors playing a role in determining TH1 or TH 2 differentiation have been proposed, including the affinity of the interaction of the TcR with the MHC II–associated peptide, the concentration of MHC-peptide complexes on the cell membrane, and signals dependent on cell-cell interactions (Table 4.2).
Cell-cell contact also plays a significant role in promoting B-cell activation by deliv-ering co-stimulatory signals to the B cell and/or by allowing direct traffic of unknown fac-tors from helper T lymphocytes to B lymphocytes. Transient conjugation between T and B lymphocytes seems to occur constantly due to the expression of complementary CAMs on their membranes. For example, T cells express CD2 and CD4, and B cells express the re-spective ligands, CD58 (LFA-3) and MHC II; both T and B lymphocytes express ICAM-1 and LFA-1, which can reciprocally interact.
The continuing proliferation and differentiation of B cells into plasma cells is assisted by several soluble factors, including IL-4, released by TH 2 cells, and IL-6 and IL-14 (B-cell growth factor), released by T lymphocytes and accessory cells. At the end of an im-mune response, the total number of antigen-specific T- and B-lymphocyte clones will re-main the same, but the number of cells in those clones will be increased severalfold. The increased residual population of antigen-specific T cells is long-lived and is believed to be responsible for the phenomenon known as immunological memory.
Related Topics