Chapter: Biology of Disease: Transfusion and Transplantation
Role of Complement in Transfusion Reactions
ROLE OF COMPLEMENT IN TRANSFUSION
REACTIONS
The activation of complement is also involved in some
forms of immunological hypersensitivity and can cause some of the prob-lems
associated with autoimmune disease . However, given that it amplifies the
actions of antibodies, complement can cause many of the problems associated
with transfusion reactions. Thus, complement causes lysis of sensitized
erythrocytes, that is, erythrocytes coated with anti-erythrocyte antibodies.
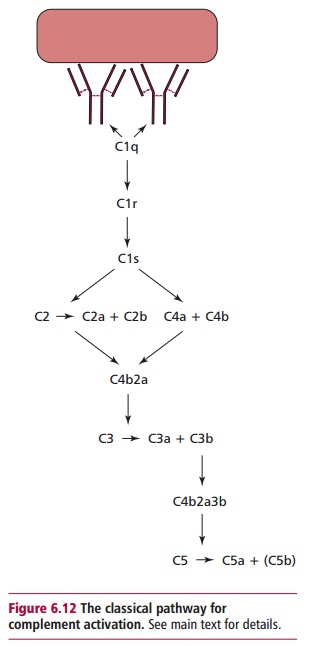
The classical pathway for complement activation is
initiated when IgG or IgM binds to an epitope, in this case on the
erythrocyte’s membrane. The antibody could be IgM, as is usually the case with
Anti-A or Anti-B, or IgG, as is the case with antibodies to Rh antigens. The
binding of antibody to the epitope induces a conformational change in the Fc
region of IgG or IgM, allowing the binding of C1 protein. The C1 is comprised
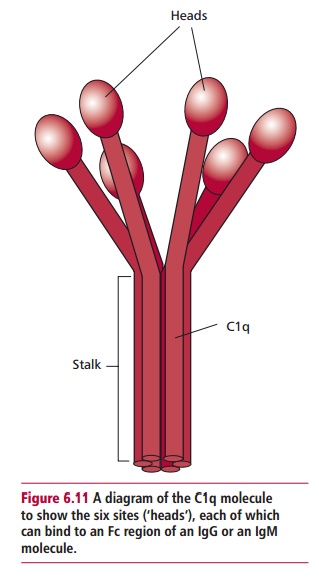
of three loosely associated pro-teins called C1q, C1r and C1s. The C1q is a large
protein and has several bind-ing sites allowing it to bind to multiple Fc
regions of antibodies (Figure 6.11).
It requires at least two of these sites to bind to adjacent Fc regions on the
sur-face of a cell to activate complement. For this reason IgM, which has
several Fc regions per molecule, is more efficient than IgG at activating
complement. Indeed, a single molecule of IgM can activate complement, whereas
it takes about 1000 molecules of IgG to achieve the density required for
activation. The binding of C1q activates C1r and this, in turn, activates C1s
which acquires proteolytic activity (Figure
6.12). C1s has two substrates: C4 and C2 each of which are hydrolyzed to
two fragments: C4a, C4b and C2a and C2b respec-tively. Proteins C4b and C2a are
the larger fragments in each case and they combine to form a new proteolytic
enzyme, C4b2a. A single enzyme molecule can generate a number of product
molecules. Thus for a limited number of antibody molecules, many molecules of
C4b2a are formed, because enzymic steps allow amplification to occur. C4b2a is
the classical pathway C3 conver-tase which
cleaves C3 into two fragments: a larger C3b moleculeand a C3a. The former binds
to the target cell membrane where it may bind a molecule of the C3 convertase
to form a C5 convertase, which
catalyzes the hydrolysis of C5 into C5a and C5b. C5b binds to the cell membrane
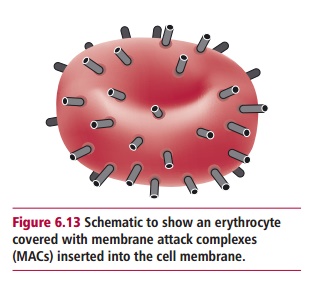
and forms a site for the build up of the Membrane
Attack Complex (MAC). This is a
large, cylindrical, hydrophobic structure constructed from single molecules of
C5b, C6, C7, C8 and several molecules of C9. When it inserts into the mem-brane
it forms a pore of approximately 10 nm diameter. Since amplification has
occurred at each enzymic step, the target cell membrane may be covered with
MACs (Figure 6.13). The MACs allow
small ions to equilibrate across the cell membrane, increasing the osmotic
pressure within the cell so that water moves across the membrane into the cell
causing it to lyze. In vitro this can
be seen as a sudden clearing of the cloudy suspension of erythrocytes. Invivo, several regulatory proteins may
prevent direct lysis of the erythrocytes.Instead, the cells are lyzed by
phagocytic cells that have receptors for C3b and other complement proteins on
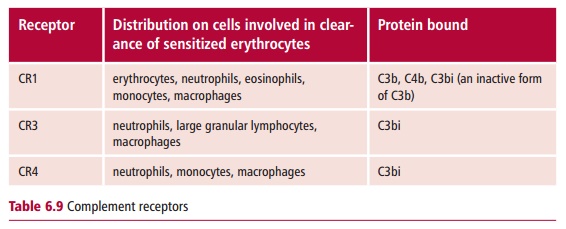
their membranes. Table 6.9 lists some
of the receptors involved in the clearance of sensitized erythrocytes. In the
transfu-sion laboratory it is much easier to look for complement proteins on
erythro-cytes than to look for antibodies, since a small amount of antibody may
result in large amounts of complement on the cells. Thus, the presence of
comple-ment proteins on cells is used as an indicator of the presence of
complement binding antibodies.
It is essential for the transfusion scientist to be able to detect hemolytic anti-bodies. Such antibodies may be present due to transfusion reactions, to HDN or they may be autoantibodies to erythrocytes, as happens in autoimmune hemolytic anemia.
samples of serum by hemolysis in vitro using the complement activity in the serum or they ma The presence of relevant antibodies may be detected in y be detected by examining the surface of erythrocytes for activated complement proteins. The presence of complement in serum dimin-ishes with storage, therefore it is recommended that samples of sera should be stored at –20ºC to retain activity if they are to be used for hemolysis deter-mination. Further, some anticoagulants, such as EDTA, inhibit complement, which may be significant if plasma rather than serum is available. Other sera may have ‘anticomplementary’ activity due to the presence of a denatured form of complement known as complementoid.
Receptors for C3b are found on all the major types of
phagocytic cells and also on erythrocytes themselves, so that even these cells
have a role in the clear-ance of immune complexes from the blood.
Antigen–antibody complexes coated in C3b bind to erythrocytes and are removed
by macrophages in the spleen and liver.
In vivo, other
complement proteins trigger inflammatory reactions. For exam-ple, C3a, C4a and
C5a cause blood basophils and tissue mast cells to degranu-late. The mediators
released stimulate inflammation , which may have consequences in a patient who
has anti-erythrocyte antibodies. In addi-tion, both C3a and C5a are chemotactic
factors for neutrophils and promote a build up of these cells, which may itself
lead to clinical problems.
The alternative pathway for complement activation is
a positive feedback loop which is usually initiated by microorganisms such as
bacteria and yeasts. However, feedback may utilize C3b, produced in the
classical pathway, and amplify the amount of C3b produced. The positive
feedback loop is control-led to prevent an overproduction of C3b. One
regulatory step binds C3b to a plasma protein called Factor H and in the bound form
is inactivated by Factor I, which converts it to C3bi, a form that can no
longer enter the amplification loop. C3b may then be degraded into smaller
fragments, C3dg and C3d, which may remain bound to the erythrocyte membrane.
The presence of natural regulators means that many antibodies that are
potentially able to lyze eryth-rocytes are unable to do so in vitro and the transfusion scientist may look for the presence of
C3d on erythrocytes to determine whether antibodies to them are present.
Related Topics