Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Special habitats and special adaptations
Physical factors affecting the deep sea - Diversity of Fishes
Physical factors affecting the deep sea
Pressure
The weight of the overlying column of water, measured in atmospheres, increases constantly with depth at a rate of l atm/10 m of descent (1 atm =1.03 kg/cm2 or 14.7 lbs/in2). Thus between the top of the mesopelagic region at 200 m and the lower bathypelagic region at 4000 m, pressure increases 20-fold, from 20 to 400 atm. The deepest living fishes, the neobythitine cusk-eels, Bassogigas profundissimus and Abyssobrotula galatheae, have been collected at 7160 and 8370 m, respectively, where they would experience pressures of 700–800 atm, or c. 12,000 lbs/in2 (Nielsen & Munk 1964; Nielsen 1977). Below the surface, pressure at any given depth is constant and predictable, whereas at the surface it can change rapidly and significantly with each passing wave.
The tremendous pressures of the deep sea do not create problems for most biological structures because fishes are made up primarily of water and dissolved minerals, which are relatively incompressible. However, pressure has an in - fluence on the volume of water molecules, water-containing compounds, and proteins, which affects the rates of chemical reactions. Several deep mesopelagic and bathypelagic species have evolved proteins that are much less sensi
Table 18.1
Representative teleostean taxa from the three major deepsea habitat types. The approximate number of deepsea families is given in parentheses the first time a group is listed. Based on Marshall (1971, 1980); Wheeler (1975); Gage and Tyler (1991); Nelson (2006). Figures from Marshall (1971), used with permission.

Gas-containing structures are parti cularly affected because both volume relationships and gas solubility are sensitive to pressure. The organ most affected is the gas bladder because it is difficult to secrete gas into a gas-filled bladder under high pressure. Three trends occur in the gas bladders of deepsea fishes that reflect the constraints of pressure:
1 The effi ciency of gas secretion depends on the interchange surface of the capillaries of the rete mirabile, the main gas-secreting organ (see Buoyancy regulation). Whereas the retes of epipelagic fishes are usually less than 1 mm long, retes of upper mesopelagic fishes are 1–2 mm long, those of lower mesopelagic fishes are 3–7 mm long, and those of some bathypelagic fishes are 15–20 mm long.
2 Although mesopelagic fishes have large gas-filled bladders, most bathypelagic fishes have lost their gas bladders. Flotation might therefore be a problem for these fishes, but their body musculature and skeletons are reduced as energy saving mechanisms and they consequently approach neutral buoyancy. As long as a fish remains at relatively constant depths, it has
minimal need for buoyancy control. However, many mesopelagic fishes undergo diurnal vertical migrations, have a greater need to adjust their buoyancy, and have retained their gas bladders. Deep benthopelagic fishes are able to hover just above the bottom with minimal energy expenditure via a different mechanism. Instead of trying to secrete gases against incredible pressure gradients, they have evolved lipid-filled gas bladders. Lipids are relatively incompressible and are lighter than sea water and thus provide flotation. Interestingly, the larvae of these fishes have gas-filled bladders, but these larvae, and the larvae of nearly all deepsea fishes, are epipelagic, where the costs of gas secretion and buoyancy adjustment are much less. Benthopelagic squaloid sharks such as Centroscymnus and Etmopterus show parallel evolution. These deepsea sharks have exceptionally large livers that account for 25% of their total body mass. Their livers contain large quantities of the low-density lipid squalene. Deepwater holocephalans also achieve neutral buoyancy via squalene and by reduced calcifi cation of their cartilaginous skeletons (Bone et al. 1995).
3 Most deepsea fishes belong to the relatively primitive teleostean superorders Protacanthopterygii, Stenopterygii, Cyclosquamata, and Scopelomorpha. These taxa typically have a direct, physostomous connection between the gas bladder and the gut. Deepsea fishes are, however, “secondarily” physoclistous, having closed the pneumatic duct, thus preventing gas from escaping out the mouth.
Temperature
At the surface, temperature is highly discontinuous, changing markedly both seasonally and daily. In the deep sea, temperature is a predictable function of depth. Surface waters are warmer than deeper waters. Water temperature declines with depth through the mesopelagic region across a permanent thermocline until one reaches the bathypelagic region, where temperature remains a relatively constant 2–5°C, depending on depth. Temperature is a strong predictor of distribution for different taxa of deepsea fishes. Ceratioid anglerfishes and darkly colored species of the bristlemouths (Cyclothone) are restricted to the deeper region. Even within the mesopelagic zone, species sort out by temperature. Hatchetfishes, pale Cyclothone, and malacosteine loosejaws are restricted to the lower half at temperatures between 5 and 10°C, whereas lanternfishes and astronesthine and melanostomiatine stomiiforms occur in the upper half at 10–20°C. Latitudinal differences in temperature–depth relationships lead to distributional differences within species. Some species such as ceratioid anglers that are mesopelagic at high latitudes occur in bathypelagic waters at lower latitudes, a phenomenon known as tropical submergence that results from the warmer surface temperatures in the tropics.
Since temperature remains fairly constant at any given depth, absolute temperature is a minimal constraint on a fish that does not move vertically. But vertically migrating mesopelagic species must swim through and function across a temperature range of as much as 20°C (see Fig. 18.1). Lanternfish species that migrate vertically have larger amounts of DNA per cell than do species that are non-migratory. Increased DNA could potentially allow for multiple enzyme systems that function at the different temperatures encountered by the fishes (Ebeling et al. 1971).
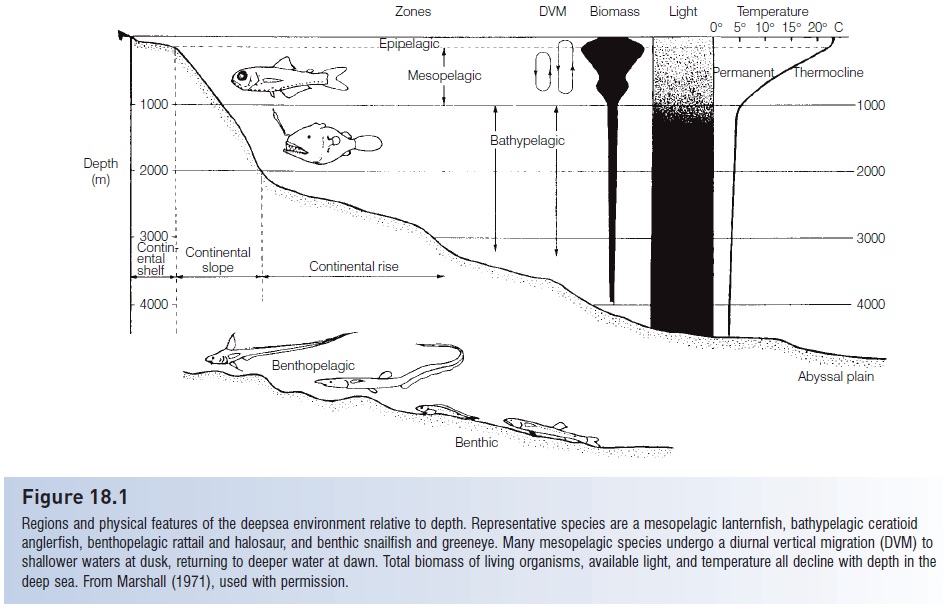
Space
The volume occupied by the deep sea is immense. Approximately 70% of the earth’s surface is covered by ocean, and 90% of the surface of the ocean overlies water deeper than 1000 m. The bathypelagic region, which makes up 75% of the ocean, is therefore the largest habitat type on earth. This large volume creates problems of finding food, conspecifics, and mates because bathypelagic fishes are never abundant. Life in the bathypelagos is extremely dilute. For example, female ceratioid anglerfishes are distributed at a density of about one per 800,000 m3, which means a male anglerfish is searching for an object the size of a football in a space about the size of a large, totally darkened football stadium.
Deepsea fishes show numerous adaptations that reflect the difficulties of finding potential mates that are widely distributed in a dark expanse. Unlike most shallow water forms, many deepsea fishes are sexually dimorphic in ways directly associated with mate localization. Mesopelagic fishes, such as lanternfishes and stomiiforms, have speciesspecific and sex-specific patterns and sizes of light organs, structures that first assure that individuals associate with the right species and then that the sexes can tell one another apart. Among benthopelagic taxa, such as macrourids, brotulids, and morids, males often have larger muscles attached to their gas bladders that are likely used to vibrate the bladder and produce sounds that can attract females from a considerable distance.
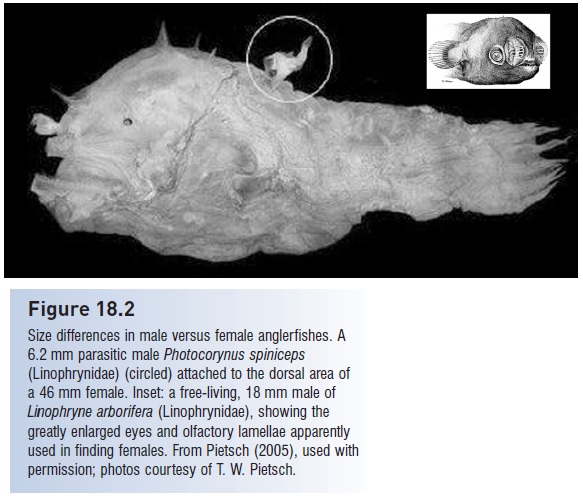
Figure 18.2
Size differences in male versus female anglerfishes. A 6.2 mm parasitic male Photocorynus spiniceps (Linophrynidae) (circled) attached to the dorsal area of a 46 mm female. Inset: a free-living, 18 mm male of Linophryne arborifera (Linophrynidae), showing the
greatly enlarged eyes and olfactory lamellae apparently used in finding females. From Pietsch (2005), used with permission; photos courtesy of T. W. Pietsch.
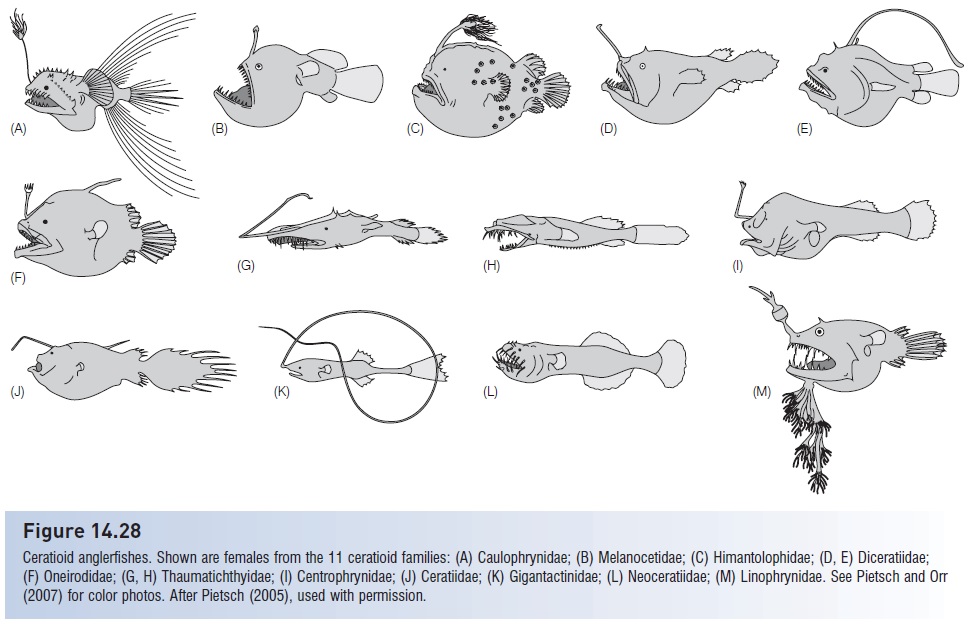
Some of the most bizarre sexual dimorphisms occur among bathypelagic species, where problems of mate localization are acute. The most speciose group of bathypelagic fishes is the ceratioid anglerfishes, of which there are 11 families and about 162 species (Bertelsen 1951; Pietsch 1976, 2005; Nelson 2006; Pietsch & Orr 2007; see Fig. 14.28). In several families, the males are dwarfed, reaching only 20–40 mm long, whereas females attain lengths 10 or more times that size, up to 1.2 m in one species. In five families, males attach temporarily to females, spawning occurs, and the males swim free (Pietsch 2005).
In five other families, the males are entirely and permanently parasitic on the females, and males in these taxa may be as small as 6.2 mm, making them the smallest known sexually mature vertebrate (Fig. 18.2). Males attach most frequently to the ventral midline of the belly of the female, but may be attached on the sides, backs, head, and even the fishing lure of a female; as many as eight males have been found attached to a single female (including some species mismatches). In parasitic species, males attach by the mouth, his mouth tissue fuses with her skin, and he becomes parasitically dependent on her for nutrition. Many of his internal organs degenerate, with the exception of his testes, which can take up more than half of his coelom. Females do not mature sexually until a male attaches to them (Pietsch 2005).
The premium placed on locating a female is reflected throughout the anatomy and physiology of searching males. During this phase, males have highly lamellated olfactory organs and well-developed olfactory tracts, bulbs, and forebrains, whereas females have almost entirely degenerate olfactory systems. Males also have extensive red muscle fi bers, the kind used for sustained swimming. Females have predominantly white muscle fi bers, which usually function for short bursts of swimming. Males of some species possess enlarged, tubular eyes that are extremely sensitive to light (see below), whereas females have small, relatively insensitive eyes. Males also have high lipid reserves in their livers, which they need because their jaw teeth become replaced by beaklike denticles that are useless for feeding but are apparently specialized for holding onto a female (the denticular jaws are derived embryologically from the same structures that in females develop into the fishing lure, discussed below; Munk 2000). All this comparative evidence indicates that males are adapted for swimming over large expanses of ocean, searching for the luminescent glow and some olfactory cue emitted by females. Females in contrast are floating relatively passively, using their bioluminescent lures to attract prey at which they make sudden lunges, and trailing pheromones through the still waters. The coevolved nature of these traits is evident from the dependence of both sexes on locating each other. Neither sex matures until the male attaches to the female.
Convergence occurs in the unrelated bathypelagic bristlemouths, which are probably the most abundant vertebrates on earth. Again, males are smaller than females, have a well-developed olfactory apparatus, extensive red muscle fi bers, and larger livers and fat reserves. Although the males are not parasitic on the females, they are unusual in that they are protandrous hermaphrodites, meaning that an individual matures first as a male and then later switches sex and becomes a female. Sex change theory predicts just such a switch because relative fitness favors being a male when small and a female when large (see Determination, differentiation, and maturation;, Gender roles in fishes). Cetomimid whalefishes – one of the few percomorph groups to occupy the bathypelagic region and second only to oneirodid anglerfishes in diversity there – have also converged on having dwarf males, although male whalefishes are not known to be parasitic on the larger females (Nelson 2006).
Light
Below the euphotic zone, light is insuffi ciently strong to promote significant plant growth. Visible light to the human eye is extinguished by 200–800 m depth, even in the uniformly clear water of the mesopelagic and bathypelagic regions. Deepsea fishes are 15–30 times more sensitive to light and can detect light down to between 700 and 1300 m, depending on surface clarity. The mesopelagic region is often termed the twilight zone, whereas the bathypelagic region is continually dark. What little light that passes into the mesopelagic region has been differentially absorbed and scattered by water molecules and turbidity and is limited to relatively short, blue-green wavelengths centered on 470 nm.
The greatly reduced illumination of the mesopelagic region, and the missing light of the bathypelagic region, have produced obvious adaptations among both the eyes and photophores of fishes living there. Bathypelagic fishes live in permanent darkness and, with the exception of male ceratioid anglers, have greatly reduced eyes that probably function primarily for detecting nearby bioluminescence. Mesopelagic fishes have modifications to their eyes that generally increase their ability to capture what little ambient light is available, although different species appear to have emphasized capturing dim ambient spacelight versus brighter point sources from bioluminescence (Warrant & Locket 2004). Mesopelagic fishes have very large eyes, often measuring 50% of head length; most North American freshwater fishes have eye diameters that are only 10–20% of head length.
Mesopelagic fishes also have comparatively large pupils and lenses and lengthened eyes. Elongation results either from a space between the pupil and lens, termed theaphakic or lensless space, or from lengthening of the retinacontainingportion of the eye posterior to the lens. Aphakic spaces have evolved convergently in protacanthopterygian platytroctids and bathylagine deepsea smelts, stenopterygian loosejaws (Malacosteinae), cyclosquamate waryfishes, and scopelomorph lanternfishes, most of which live in the upper region of the mesopelagic zone. Tubular eyes, more characteristic of deeper mesopelagic species, have evolved convergently in four superorders and five orders of mesopelagic fishes, including protacanthopterygian barreleyes, stenopterygian hatchetfishes, paracanthopterygian anglerfishes, and acanthopterygian whalefishes. Eye elongation provides two visual benefits, increasing the sensitivity of the eye to light by about 10% and also increasing binocular overlap, which aids depth perception (Marshall 1971; Lockett 1977).
Mesopelagic fishes have pure rod retinae with visual pigments that are maximally sensitive at about 470 nm, which is a good match to the light environment at mesopelagic depths and also matches the light output from photophores, structures that are much more common among mesopelagic than bathypelagic fishes. Bioluminescence has evolved independently in at least five superorders of deepsea teleosts – protacanthopterygians, stenopterygians, scopelomorphs, paracanthopterygians, and acanthopterygians – as well as in dogfish sharks, squids, crustaceans, and other invertebrates. Light organs, in addition to identifying the species and sex of the emitter, may also illuminate nearby prey. The structures that bioluminesce may be a simple luminescent gland backed by black skin that emits on its own or contains bioluminescent bacteria. More complex circular photophores may be backed by silvery reflective material with a lens through which light passes. In highly derived photophores, the lens may be pigmented and hence the light transmitted is of a different wavelength, as in the malacosteine loosejaws which have a red filter over the subocular photophores and also have retinal reflectors and receptors sensitive to red wavelengths (e.g., Herring & Cope 2005). This unique combination of luminescent emission and spectral sensitivity could give loosejaws a private channel over which they can communicate without being detected by potential predators or prey. It could also serve to maximize illumination of red mesopelagic crustaceans (Lockett 1977; Denton et al. 1985; Sutton 2005). Photophores tend to flash on for 0.2–4 s, depending on species. Different species of lanternfishes may have similar photophore patterns but different flash rates, suggesting a convergence in communication tactics between deepsea fishes and fi refl ies (Meinsinger & Case 1990).
Food
Limited light and huge volume mean that food is extremely scarce in the deep sea. All marine food chains, except at thermal vents, originate in the euphotic zone, which makes up only 3% of the ocean. Food for bathypelagic fishes must therefore first pass through the filter of vertebrates, invertebrates, and bacteria in the mesopelagic zone; much of this food rains down weakly, unpredictably, and patchily in the form of carcasses, sinking sargassum weed, detritus, and feces. All deepsea fishes are carnivorous, feeding either on zooplankton, larger invertebrates, or other fishes. Zooplankton biomass at the top of the bathypelagos is only about 1% of what it is at the surface, and densities of benthic invertebrates decrease with depth and distance from continental shores. High densities, diversities, and productivity of invertebrates at thermal vents on the deepsea floor do not support a similar abundance or diversity of fishes. Only three species – a bythitid brotula and two zoarcid eel-pouts – are endemic to and frequent vent areas (Grassle 1986; Cohen et al. 1990). A general scarcity of food in the deep sea puts a premium on both saving and obtaining energy. Convergent traits in both categories are readily apparent.
Foraging adaptations
Deepsea fishes show a number of convergent foraging traits (Gartner et al. 1997). In general, zooplanktivores have small mouths and numerous, relatively fine gill rakers, whereas predators on larger animals have larger mouths and fewer, coarser gill rakers. Daggerlike teeth or some other form of long, sharp dentition is so characteristic of deepsea forms that their family names often refer directly or indirectly to this trait, including such colorfully named groups as dragonfishes, daggertooths, bristlemouths, snag gletooths, viperfishes, sabretooths, and fangtooths. Large, expandable mouths, hinged jaws, or distensible stomachs are also reflected in such names as gulpers, swallowers, and loosejaws. Saccopharyngoid gulper and swallower eels have enormous mouths that can expand to >10 times the volume of the animal’s entire body, the largest mouth : body volume of any known vertebrate (Nielsen et al. 1989). Black dragonfishes, viperfishes, ceratioid anglerfishes, and sabertooth fishes can swallow prey larger than themselves (Fig. 18.3),
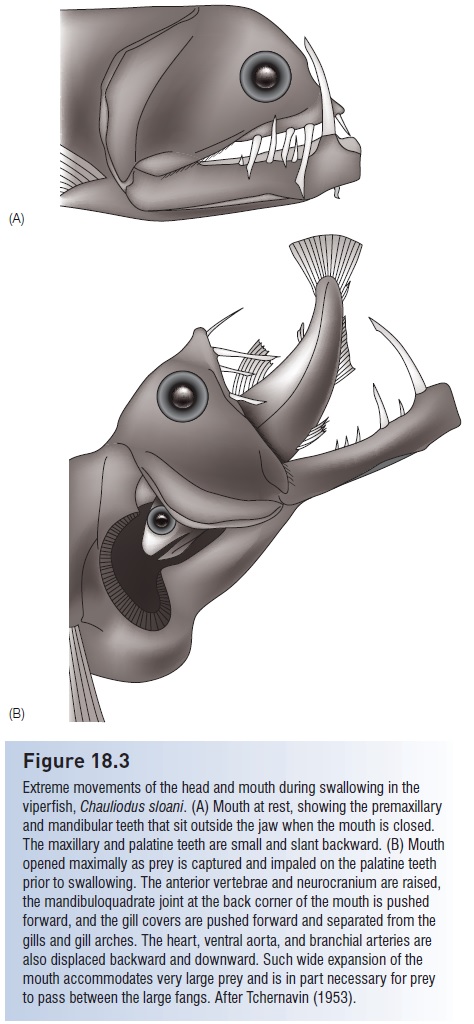
Figure 18.3
Extreme movements of the head and mouth during swallowing in the viperfish, Chauliodus sloani. (A) Mouth at rest, showing the premaxillary and mandibular teeth that sit outside the jaw when the mouth is closed. The maxillary and palatine teeth are small and slant backward. (B) Mouth opened maximally as prey is captured and impaled on the palatine teeth prior to swallowing. The anterior vertebrae and neurocranium are raised, the mandibuloquadrate joint at the back corner of the mouth is pushed forward, and the gill covers are pushed forward and separated from the gills and gill arches. The heart, ventral aorta, and branchial arteries are also displaced backward and downward. Such wide expansion of the mouth accommodates very large prey and is in part necessary for prey to pass between the large fangs. After Tchernavin (1953).
as much as three times so in the case of the anglerfishes. Their swallowing abilities are increased because the pectoral girdle is disconnected from the skull, enlarging the intercleithral space of the throat (see Pharyngeal jaws). All of these anatomical specializations point to a strategy of taking advantage of any feeding opportunity that may come along, despite the size of the prey. A small number of shallow water paracanthopterygian species, notably the goosefishes, frogfishes, batfishes, and anglerfishes, possess modified dorsal spines that are waved in front of prey species to lure them within striking distance. Such lures reach their greatest and most diverse development among mesopelagic and bathypelagic fishes, where they occur on viperfishes, various dragonfishes, astronesthine snaggletooths, most ceratioid anglerfishes, and arguably as luminescent organs in the mouths of hatchetfishes, lanternfishes, and some anglerfishes and on the illuminated tail tip of the gulper eels. The typical anglerfish lure consists of an elongate dorsal spine, the illicium, tipped by an expanded structure called the esca (Fig. 18.4). Escae tend to have species specific shapes, can regenerate if damaged, and are moved in a variety of motions that imitate the swimming of a small fish or shrimp (see Pietsch 1974).
Most mesopelagic fishes undertake evening migrations from the relatively unproductive mesopelagic region to the richer epipelagic zone to feed; they then return to the mesopelagic region at dawn (see Fig. 18.1). The migration involves movements to near the surface from as deep as 700 m, can take an hour or more, and may entail considerable energy expenditure. This movement is so characteristic of mesopelagic fishes, crustaceans, and mollusks that the community of organisms that migrates is referred to as the deep scattering layer, whose presence is discernible on sonar screens because of reflection of sonar signals off the
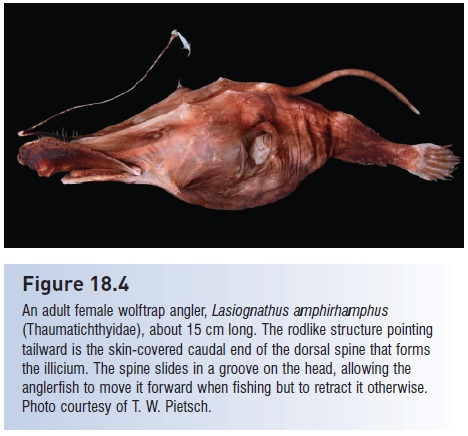
Figure 18.4
An adult female wolftrap angler, Lasiognathus amphirhamphus (Thaumatichthyidae), about 15 cm long. The rodlike structure pointing tailward is the skin-covered caudal end of the dorsal spine that forms the illicium. The spine slides in a groove on the head, allowing the anglerfish to move it forward when fishing but to retract it otherwise. Photo courtesy of T. W. Pietsch.
gas bladders of the fishes. Hypotheses about the adaptiveness of the migration include: (i) a net energy gain from feeding in warm water and metabolizing in cold water; and (ii) exploiting surface currents that bring new food into the water column above the migrator. It is apparent that the migration serves a foraging purpose, given the 100-fold difference in plankton biomass between the two regions and also given that stomachs of migrators are empty in the evening before migration and full in the morning after migration.
It is in the deeper region of the bathypelagos that we find the most extreme adaptations for opportunistic prey capture and energy conservation. Bathypelagic fishes remain in place, perhaps because external cues of changing daylight are lacking or the energetic costs of migrating are too high. They instead lure prey with bioluminescent lures. Observations from submersibles suggest that bathypelagic forms adapt a “fl oat-and-wait” foraging mode, hovering relatively motionless in the water column and making quick lunges at prey. This motionless hovering and luring even occurs when purportedly bathypelagic anglerfish forage near the bottom, as evidenced by fortuitous observations of a Whipnose Anglerfish, Gigantactis, swimming slowly upsidedown just off the bottom, its illicium held stiffly in front in a slight downward-pointing arc (Moore 2002) (Fig. 18.5).

Figure 18.5
A 50 cm long Whipnose Anglerfish presumably foraging just above the bottom at 5000 m depth. Its illicial lure is extended down toward the bottom (lower two profiles are shadows cast by photographic lights). Interestingly, in gigantactinids, the teeth of the lower jaw are elongated and curved, much like the upper jaw teeth of other anglerfishes, implying that upside-down foraging may be common in Whipnose Anglerfishes. From Moore (2002), used with permission.
Energy conservation
Deepsea fishes minimize their daily and long-term expenditure of calories in many ways. Biochemically, rates of enzymatic and metabolic activity and even levels of adenosine triphosphate (ATP) generating enzymes are lower in deepsea fishes than in shallow water relatives, which conserves energy used in locomotion, osmotic regulation, and protein synthesis (Somero et al. 1991). Energy savings are also accomplished via elimination or replacement of heavy components. Structurally, bathypelagic fishes are fragile compared with shallow water, mesopelagic, and even deepsea benthic fishes. Many of the heavy bony elements of shallow water relatives have been eliminated. Pelvic fins are often missing or reduced to rudiments, bones of the head are reduced to thin strands, and many species are scaleless. Spines are rare among deepsea fishes; even the few acanthopterygian groups that have managed to invade the deep sea, such as melamphaid bigscale fishes and chiasmodontid swallowers, have very feeble fin spines. Body musculature is also greatly reduced, by as much as 95% in the trunk and caudal regions compared with shallow water forms.
Lacking trunk musculature, predator evasion becomes a problem. Most deepsea fishes are colored in ways that should minimize their detection by potential predators. Mesopelagic fishes tend to be silvery or brown with ventral photophores that point downward. Silvery fishes disappear in open water (see Invisible fishes). Ventral photophores may aid in breaking up the silhouette of the fish when viewed from below against the backdrop of weak downwelling light (Johnsen et al. 2004). Bathypelagic fishes are generally dark brown or black, as would be expected where the background is black. Additional energy savings are attained by replacing heavy structural components with less dense substances. Where glycerol lipids occur in shallow water fishes, deepsea forms have less dense waxy esters. These structural changes save energy because metabolic costs of both construction and maintenance are reduced. In addition, elimination and replacement of heavy elements reduces the mass of the fish, making it closer to neutral buoyancy and eliminating costs associated with fi ghting gravity.
Bathypelagic fishes as a group tend to have free neuromasts in their lateral lines, rather than having lateral line organs contained in canals, as in mesopelagic and benthic groups. Free neuromasts in shallow water fishes, such as goosefishes, cavefishes, and many gobies, are usually associated with a very sedentary life style, again suggesting a premium on energy-conserving tactics and an ability to detect minor water disturbances among bathypelagic species.
Related Topics