Chapter: Basic & Clinical Pharmacology : Antiviral Agents
New & Investigational Antiretroviral Agents
NEW & INVESTIGATIONAL ANTIRETROVIRAL AGENTS
New therapies are
continually being sought that exploit other HIV targets, have activity against
resistant viral strains, have a lower incidence of adverse effects, and offer
convenient dosing. Newly approved agents and those currently in advanced stages
of clinical development include the NRTI agents elvucitabine,racivir, and
apricitabine; the NNRTI agent
rilpivirine; entryinhibitors such as the CCR5 receptor antagonists vicriviroc and PRO 140, the fusion inhibitor TNX-355
(ibalizumab); and integrase inhibitors such as elvitegravir. In addition, new drug classes such as maturation
inhibitors (bevirimat) are under
investigation.
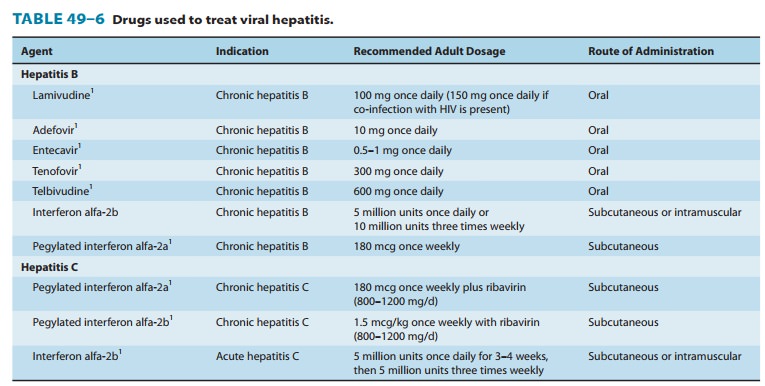
ANTIHEPATITIS AGENTS
Several agents
effective against HBV and HCV are now available (Table 49–6). Current treatment
is suppressive rather than cura-tive and the high prevalence of these
infections worldwide, with their concomitant morbidity and mortality, reflect a
critical need for improved therapeutics.
INTERFERON ALFA
Interferons are host
cytokines that exert complex antiviral, immuno-modulatory, and
antiproliferative actions . Interferon alfa appears to function by induction of
intracellular signals following binding to specific cell membrane receptors,
resulting in inhibition of viral penetration, translation, transcrip-tion,
protein processing, maturation, and release, as well as increased host
expression of major histocompatibility complex antigens, enhanced phagocytic
activity of macrophages, and aug-mentation of the proliferation and survival of
cytotoxic T cells.
Injectable
preparations of interferon alfa are available for treat-ment of both HBV and
HCV infections (Table 49–6). Interferon alfa-2a and interferon alfa-2b may be
administered either subcu-taneously or intramuscularly; interferon alfacon-1 is
administered subcutaneously. Elimination half-life is 2–5 hours for interferon
alfa-2a and -2b, depending on the route of administration. The half-life of
interferon alfacon-1 in patients with chronic HCV ranges from 6 to 10 hours.
Alfa interferons are filtered at the glomerulus and undergo rapid proteolytic
degradation during tubular reabsorption, such that detection in the systemic
circula-tion is negligible. Liver metabolism and subsequent biliary excre-tion
are considered minor pathways.
A meta-analysis of clinical trials in patients with chronic HBV infection showed that treatment with interferon alfa is associated with a higher incidence of hepatitis e antigen (HBeAg) serocon-version and undetectable HBV DNA levels compared with placebo. The addition of the pegylated moiety results in further increases in the proportion of patients with HBeAg seroconver-sion (∼ 30%) and a decline by approximately 4 log copies/mL (a 99.99% reduction) in HBV DNA after 1 year.
The use of pegylated
(polyethylene glycol-complexed) interferon alfa-2a and pegylated interferon
alfa-2b results in slower clearance and longer terminal half-lives and steadier
concentrations, which allows for less frequent dosing in patients with chronic
HCV infec-tion. Renal elimination accounts for about 30% of clearance, and
clearance is approximately halved in subjects with impaired renal function;
dosage must therefore be adjusted.
The
adverse effects of interferon alfa include a flu-like syndrome (ie, headache,
fevers, chills, myalgias, and malaise) that typically occurs within 6 hours after
dosing; this syndrome occurs in more than 30% of patients during the first week
of therapy and tends to resolve upon continued administration. Transient
hepatic enzyme elevations may occur in the first 8–12 weeks of therapy and
appear to be more common in responders. Potential adverse effects during
chronic therapy include neuro-toxicities (mood disorders, depression,
somnolence, confusion, seizures), myelosuppression, profound fatigue, weight
loss, rash, cough, myalgia, alopecia, tinnitus, reversible hearing loss,
retin-opathy, pneumonitis, and possibly cardiotoxicity. Induction of
autoantibodies may occur, causing exacerbation or unmasking of autoimmune
disease (particularly thyroiditis). The polyethylene glycol molecule is a
nontoxic polymer that is readily excreted in the urine.
Contraindications
to interferon alfa therapy include hepatic de-compensation, autoimmune disease,
and history of cardiac arrhyth-mia. Caution is advised in the setting of
psychiatric disease, epilepsy, thyroid disease, ischemic cardiac disease,
severe renal insufficiency, and cytopenia. Alfa interferons are abortifacient
in primates and should not be administered in pregnancy. Potential drug-drug
interac-tions include increased theophylline and methadone levels. Co-administration
with didanosine is not recommended because of a risk of hepatic failure, and
co-administration with zidovudine may exacerbate cytopenias.
Related Topics