Chapter: Medicine and surgery: Genitourinary system
Membranous glomerulonephritis - Glomerular disease
Membranous glomerulonephritis
Definition
This is the one of the two most common causes of nephrotic syndrome in non-diabetic adults (together with focal segmental glomerulosclerosis). Also called membranous nephropathy (MN), glomerulonephropathy or glomerulopathy.
Incidence/prevalence
MN accounts for as many as 25ŌĆō30% of cases of adult nephrotic syndrome. The idiopathic form causes Ōł╝20% of cases, although this varies by population.
Age
Peak age 30ŌĆō50 years.
Aetiology
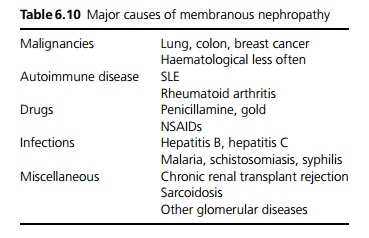
Eighty-five per cent are idiopathic. Secondary causes are shown in Table 6.10. Malignancies are an important cause ŌĆō being present in 5ŌĆō10% of cases, particularly in older persons.
Pathophysiology
The mechanism is unknown. It is thought that antibodies directed against antigens in the subepithelial space form immune complexes. In idiopathic MN, these are probably autoantibodies, whereas in secondary causes circulating antigen is filtered by the kidney, leading to de novo immune complexes or possibly circulating immune complexes. Because the immune deposits are subepithelial there is usually no marked inflammatory response. The BM becomes more permeable to protein, leading to proteinuria and the nephrotic syndrome. Over many years, there is increase in mesangial matrix causing hyalinization of glomeruli and loss of nephrons.
Clinical features
Patients may present with asymptomatic proteinuria, or (in most cases) nephrotic syndrome. There may be features consistent with an underlying disease, particularly malignancies, which is usually overt at time of presentation.
Macroscopy/microscopy
Renal biopsy shows thickened capillary loops (ranging from mild in early disease, to marked in late disease), usually with mild to moderate mesangial proliferation. Silver stains classically show ŌĆśspikesŌĆÖ where basement membrane has grown between subepithelial deposits. Immunofluorescence shows immune complexes (IgG, IgM, C3) deposited subepithelially in the BM in a diffuse global pattern.
Investigations
Renal biopsy is required for diagnosis. Complement is normal (low levels in SLE-associated and hepatitis B-associated MN).
Management
Any underlying cause should be treated. Where no cause has been identified, treatment is disputed, as the course is often slow and spontaneous remission may occur. There is evidence that intensive immunosuppression with cor-ticosteroids and chlorambucil or cyclophosphamide for 6 months improves prognosis, but this has a significant risk of adverse effects. Other treatments include ACE inhibitors, NSAIDs and omega-3 fatty acids (fish oils).
Prognosis
Approximately 50% develop CRF over 10 years; 25% undergo remission; 25% have stable, persistent proteinuria. Some patients develop a rapidly progressive course to end-stage renal failure.
Related Topics