Chapter: Medicine and surgery: Genitourinary system
Goodpasture’s disease (anti-GBM disease) - Glomerular disease
Goodpasture’s disease (anti-GBM disease)
Definition
Anti-GBM disease is a rare disease characterised by the development of autoantibodies to the basement membranes of the glomerulus and lung alveoli, resulting in glomerulonephritis.
Age
Young > old
Sex
M > F
Aetiology/pathophysiology
The pathogenic antibodies have been characterised as IgG autoantibodies to the α3 chain of type IV collagen. The response to the immune deposits is complement activation, polymorph infiltration and a proliferative glomerulonephritis. Crescents form as a result of epithelial cell proliferation and monocyte infiltration into Bowman’s space, causing rapidly progressive glomerulonephritis (RPGN).
Clinical features
The usual presentation is of acute renal failure with oliguria, an active urine sediment with dysmorphic red blood cells, red cell casts and proteinuria.
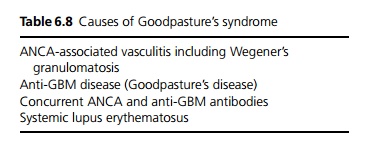
Goodpasture’s syndrome is defined as RPGN with pulmonary haemorrhage. Symptoms include haemoptysis, shortness of breath and symptoms of anaemia. Although this may occur in anti-GBM disease it is more commonly due to other causes (see Table 6.8).
Pulmonary haemorrhage is more common in patients with underlying lung disease, lung infections, pulmonary oedema and in smokers.
Macroscopy/microscopy
On renal biopsy there are diffuse global proliferative changes on light microscopy, often crescentic. Immunofluorescence demonstrates linear IgG and C3 deposits at the GBM.
Investigations
Initial investigation is as for acute renal failure and nephritic syndrome.
Serum anti-GBM antibody can be detected by radioimmunoassay (ELISA). Up to 40% of cases have a
Chest X-ray may demonstrate pulmonary infiltrates due to haemorrhage.
Pulmonary function tests may be performed to look for increased transfer factor (evidence of alveolar haemorrhage).
Management
Plasmapheresis is used to remove circulating anti-GBM, and high dose steroids and cyclophosphamide are used to switch off the production of antibody. Smoking, lung infection and pulmonary oedema all increase the risk of pulmonary haemorrhage.
If patients are dialysis dependent at presentation, and have 100% crescents on renal biopsy, the chances of renal recovery are poor. The decision to treat these patients if they have no evidence of pulmonary haemorrhage or other vasculitis with aggressive therapy is difficult, as the risks of the above treatment are considerable.
Anti-GBM disease only rarely relapses, so the cyclophosphamide can be stopped after 3 months, and the steroids are tailed off.
Prognosis
Patient survival and long-term renal function correlate well with the degree of renal impairment at presentation. Relapses occur particularly in those with ANCA and anti-GBM antibody, and it may occasionally recur after transplantation. Early diagnosis and treatment is the key to reducing morbidity and mortality.
Related Topics