Chapter: Basic & Clinical Pharmacology : Agents Used in Cardiac Arrhythmias
Lidocaine (SubGroup 1B)
LIDOCAINE (SUBGROUP 1B)
Lidocaine
has a low incidence of toxicity and a high degree of effectiveness in
arrhythmias associated with acute myocardial infarction. It is used only by the
intravenous route.
Cardiac Effects
Lidocaine
blocks activated and inactivated sodium channels with rapid kinetics (Figure
14–10); the inactivated state block ensures
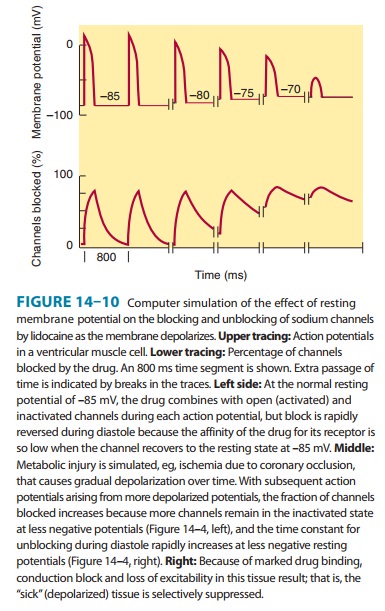
greater
effects on cells with long action potentials such as Purkinje and ventricular
cells, compared with atrial cells. The rapid kinetics at normal resting
potentials result in recovery from block between action potentials and no
effect on conduction. The increased inac-tivation and slower unbinding kinetics
result in the selective depression of conduction in depolarized cells. Little
effect is seen on the ECG in normal sinus rhythm.
Toxicity
Lidocaine
is one of the least cardiotoxic of the currently used sodium channel blockers.
Proarrhythmic effects, including SA node arrest, worsening of impaired
conduction, and ventricular arrhyth-mias, are uncommon with lidocaine use. In
large doses, especially in patients with preexisting heart failure, lidocaine
may cause hypoten-sion—partly by depressing myocardial contractility.
Lidocaine’s
most common adverse effects—like those of other local anesthetics—are
neurologic: paresthesias, tremor, nausea of central origin, lightheadedness,
hearing disturbances, slurred speech, and convulsions. These occur most commonly
in elderly or other-wise vulnerable patients or when a bolus of the drug is
given too rapidly. The effects are dose-related and usually short-lived;
seizures respond to intravenous diazepam. In general, if plasma levels above 9
mcg/mL are avoided, lidocaine is well tolerated.
Pharmacokinetics & Dosage
Because
of its extensive first-pass hepatic metabolism, only 3% of orally administered
lidocaine appears in the plasma. Thus, lido-caine must be given parenterally.
Lidocaine has a half-life of 1–2 hours. In adults, a loading dose of 150–200 mg
administered over about 15 minutes (as a single infusion or as a series of slow
boluses) should be followed by a maintenance infusion of 2–4 mg/min to achieve
a therapeutic plasma level of 2–6 mcg/mL. Determination of lidocaine plasma
levels is of great value in adjusting the infusion rate. Occasional patients
with myocardial infarction or other acute illness require (and tolerate) higher
con-centrations. This may be due to increased plasma α1-acid glyco-protein, an acute-phase reactant
protein that binds lidocaine, making less free drug available to exert its
pharmacologic effects.
In
patients with heart failure, lidocaine’s volume of distribu-tion and total body
clearance may both be decreased. Therefore, both loading and maintenance doses
should be decreased. Since these effects counterbalance each other, the
half-life may not be increased as much as predicted from clearance changes
alone. In patients with liver disease, plasma clearance is markedly reduced and
the volume of distribution is often increased; the elimina-tion half-life in
such cases may be increased threefold or more. In liver disease, the
maintenance dose should be decreased, but usual loading doses can be given.
Elimination half-life deter-mines the time to steady state. Although
steady-state concentra-tions may be achieved in 8–10 hours in normal patients
and patients with heart failure, 24–36 hours may be required in those with
liver disease. Drugs that decrease liver blood flow (eg, propranolol,
cimetidine) reduce lidocaine clearance and so increase the risk of toxicity
unless infusion rates are decreased. With infusions lasting more than 24 hours,
clearance falls and plasma concentrations rise. Renal disease has no major effect
on lidocaine disposition.
Therapeutic Use
Lidocaine
is the agent of choice for termination of ventricular tachycardia and
prevention of ventricular fibrillation after cardio-version in the setting of
acute ischemia. However, routine prophy-lactic
use of lidocaine in this setting may actually increase totalmortality,
possibly by increasing the incidence of asystole, and is not the standard of
care. Most physicians administer IV lidocaine only to patients with
arrhythmias.
Related Topics