Chapter: Basic & Clinical Pharmacology : Drugs Used in the Treatment of Gastrointestinal Diseases
Laxatives
LAXATIVES
The
overwhelming majority of people do not need laxatives; yet they are
self-prescribed by a large portion of the population. For most people,
intermittent constipation is best prevented with a high-fiber diet, adequate
fluid intake, regular exercise, and the heeding of nature’s call. Patients not
responding to dietary changes or fiber supplements should undergo medical
evaluation before initiating long-term laxative treatment. Laxatives may be
classified by their major mechanism of action, but many work through more than
one mechanism.
BULK-FORMING LAXATIVES
Bulk-forming laxatives are indigestible, hydrophilic colloids that absorb water, forming a bulky, emollient gel that distends the colon and promotes peristalsis. Common preparations include natural plant products (psyllium, methylcellulose) and synthetic fibers (polycarbophil). Bacterial digestion of plant fibers within the colon may lead to increased bloating and flatus.
STOOL SURFACTANT AGENTS (SOFTENERS)
These agents soften stool material, permitting water and lipids to penetrate. They may be administered orally or rectally. Common agents include docusate (oral or enema) and glycerin suppository. In hospitalized patients, docusate is commonly prescribed to pre-vent constipation and minimize straining. Mineral oil is a clear, viscous oil that lubricates fecal material, retarding water absorp-tion from the stool. It is used to prevent and treat fecal impaction in young children and debilitated adults. It is not palatable but may be mixed with juices. Aspiration can result in a severe lipid pneumonitis. Long-term use can impair absorption of fat-soluble vitamins (A, D, E, K).
OSMOTIC LAXATIVES
The
colon can neither concentrate nor dilute fecal fluid: fecal water is isotonic
throughout the colon. Osmotic laxatives are sol-uble but nonabsorbable
compounds that result in increased stool liquidity due to an obligate increase
in fecal fluid.
Nonabsorbable Sugars or Salts
These agents may be
used for the treatment of acute constipation or the prevention of chronic constipation.
Magnesium hydroxide(milk of magnesia) is
a commonly used osmotic laxative. Itshould not be used for prolonged periods in
patients with renal insufficiency due to the risk of hypermagnesemia. Sorbitol and lactulose are nonabsorbable sugars that can be used to prevent
ortreat chronic constipation. These sugars are metabolized by colonic bacteria,
producing severe flatus and cramps.
High doses of osmotically active agents produce prompt bowel evacuation (purgation) within 1–3 hours. The rapid movement of water into the distal small bowel and colon leads to a high volume of liquid stool followed by rapid relief of constipation. The most commonly used purgatives are magnesium citrate and sodium phosphate. Sodium phosphate is available as a nonpre-scription liquid formulation and by prescription as a tablet for-mulation. When taking these agents, it is very important that patients maintain adequate hydration by taking increased oral liquids to compensate for fecal fluid loss. Sodium phosphate fre-quently causes hyperphosphatemia, hypocalcemia, hypernatremia, and hypokalemia. Although these electrolyte abnormalities are clinically insignificant in most patients, they may lead to cardiacarrhythmias or acute renal failure due to tubular deposition of calcium phosphate (nephrocalcinosis). Sodium phosphate prepa-rations should not be used in patients who are frail or elderly, have renal insufficiency, have significant cardiac disease, or are unable to maintain adequate hydration during bowel preparation.
Balanced Polyethylene Glycol
Lavage solutions containing polyethylene glycol (PEG) are used for complete colonic cleansing before gastrointestinal endoscopic procedures. These balanced, isotonic solutions contain an inert, nonabsorbable, osmotically active sugar (PEG) with sodium sulfate, sodium chloride, sodium bicarbonate, and potassium chloride. The solution is designed so that no significant intravascular fluid or electrolyte shifts occur. Therefore, they are safe for all patients. The solution should be ingested rapidly (2–4 L over 2–4 hours) to pro-mote bowel cleansing. For treatment or prevention of chronic con-stipation, smaller doses of PEG powder may be mixed with water or juices (17 g/8 oz) and ingested daily. In contrast to sorbitol or lactu-lose, PEG does not produce significant cramps or flatus.
STIMULANT LAXATIVES
Stimulant laxatives
(cathartics) induce bowel movements through a number of poorly understood
mechanisms. These include direct stimulation of the enteric nervous system and
colonic electrolyte and fluid secretion. There has been concern that long-term
use of cathartics could lead to dependence and destruction of the myen-teric
plexus, resulting in colonic atony and dilation. More recent research suggests
that long-term use of these agents probably is safe in most patients.
Cathartics may be required on a long-term basis, especially in patients who are
neurologically impaired and in bed-bound patients in long-term care facilities.
Anthraquinone Derivatives
Aloe, senna, and cascara occur naturally in plants.
These laxa-tives are poorly absorbed and after hydrolysis in the colon,
pro-duce a bowel movement in 6–12 hours when given orally and within 2 hours
when given rectally. Chronic use leads to a charac-teristic brown pigmentation
of the colon known as “melanosis coli.” There has been some concern that these
agents may be car-cinogenic, but epidemiologic studies do not suggest a
relation to colorectal cancer.
Diphenylmethane Derivatives
Bisacodyl
is available in tablet and suppository formulations for the treatment of acute
and chronic constipation. It also is used in conjunction with PEG solutions for
colonic cleansing prior to colonoscopy. It induces a bowel movement within 6–10
hours when given orally and 30–60 minutes when taken rectally. It has minimal
systemic absorption and appears to be safe for acute and long-term use.
Phenolphthalein, another agent in this class, was removed from the market owing
to concerns about possible cardiac toxicity.
CHLORIDE CHANNEL ACTIVATOR
Lubiprostone is a prostanoic acid derivative labeled for use inchronic
constipation and irritable bowel syndrome (IBS) with pre-dominant constipation.
It acts by stimulating the type 2 chloride channel (ClC-2) in the small
intestine. This increases chloride-rich fluid secretion into the intestine,
which stimulates intestinal motil-ity and shortens intestinal transit time.
Over 50% of patients experience a bowel movement within 24 hours of taking one
dose. There appears to be no loss of efficacy with long-term therapy. After
discontinuation of the drug, constipation may return to its pretreatment
severity. Lubiprostone has minimal systemic absorp-tion but is designated
category C for pregnancy because of increased fetal loss in guinea pigs.
Lubiprostone may cause nausea in up to 30% of patients due to delayed gastric
emptying.
OPIOID RECEPTOR ANTAGONISTS
Acute and chronic
therapy with opioids may cause constipation by decreasing intestinal motility,
which results in prolonged transit time and increased absorption of fecal water
. Use of opioids after surgery for treatment of pain as well as endogenous
opioids also may prolong the duration of postoperative ileus. These effects are
mainly mediated through intestinal mu (μ)-opioid recep-tors. Two selective
antagonists of the μ-opioid receptor are com-mercially available: methylnaltrexone bromide and alvimopan. Because these agents do not
readily cross the blood-brain barrier, they inhibit peripheral μ-opioid
receptors without impacting anal-gesic effects within the central nervous
system. Methylnaltrexone is approved for the treatment of opioid-induced
constipation in patients receiving palliative care for advanced illness who
have had inadequate response to other agents. It is administered as a
subcuta-neous injection (0.15 mg/kg) every 2 days. Alvimopan is approved for
short-term use to shorten the period of postoperative ileus in hospitalized
patients who have undergone small or large bowel resection. Alvimopan (12 mg
capsule) is administered orally within 5 hours before surgery and twice daily
after surgery until bowel function has recovered, but for no more than 7 days.
Because of possible cardiovascular toxicity, alvimopan currently is restricted
to short-term use in hospitalized patients only.
SEROTONIN 5-HT4-RECEPTOR AGONISTS
Stimulation
of 5-HT4 receptors on the presynaptic
terminal of submucosal intrinsic primary afferent nerves enhances the release
of their neurotransmitters, including calcitonin gene-related pep-tide, which
stimulate second-order enteric neurons to promote the peristaltic reflex
(Figure 62–4). These enteric neurons stimulate proximal bowel contraction (via
acetylcholine and substance P) and distal bowel relaxation (via nitric oxide
and vasoactive intestinal peptide).
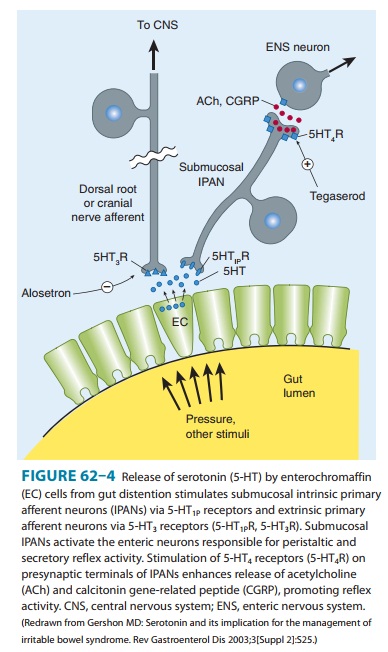
Tegaserod is a serotonin 5-HT4partial agonist that has highaffinity for 5-HT4 receptors but no appreciable binding to 5-HT3 or dopamine receptors. Tegaserod was approved for the treatmentof patients with chronic constipation and IBS with predominant constipation. Although tegaserod initially appeared to be extremely safe, it was voluntarily removed from the general market in 2007 because of an increased incidence of serious cardiovascular events. These adverse events have been attributed to inhibition of the 5-HT1B receptor. Another partial 5-HT4 agonist, cisapride, was also associated with an increased incidence of cardiovascular events that was attributed to inhibition of cardiac hERG (human ether-a-go-go-related gene) K+ channels, which resulted in QTc prolongation in some patients.
Prucalopride is a high-affinity 5-HT4agonist that is availablein Europe (but not in
the USA) for the treatment of chronic con-stipation in women. In contrast to
cisapride and tegaserod, it does not appear to have significant affinities for
either hERG channels or 5-HT1B. In three 12-week clinical trials of patients with severe
chronic constipation, it resulted in a significant increase in bowel movements
compared with placebo. The long-term efficacy and safety of this agent require
further study.
GUANYLATE CYCLASE C AGONISTS
Linaclotide is a
poorly absorbed 14-amino-acid peptide that binds to the guanylate cylase C
receptor on the luminal surface of intesti-nal enterocytes, activating the cystic
fibrosis transmembrane con-ductance channel and stimulating intestinal fluid
secretion. Preliminary results of phase 3 clinical trials confirm its efficacy
in patients with chronic constipation. Further studies are anticipated.
Related Topics