Chapter: Basic & Clinical Pharmacology : Drugs Used in the Treatment of Gastrointestinal Diseases
Anti-Tumor Necrosis Factor Therapy
ANTI-TUMOR NECROSIS FACTOR THERAPY
Pharmacokinetics & Pharmacodynamics
A dysregulation of the
helper T cell type 1 (TH1) response and
regulatory T cells (Tregs) is present in inflammatory bowel disease, especially
Crohn’s disease. One of the key proinflammatory cyto-kines in inflammatory
bowel disease is tumor necrosis factor (TNF). TNF is produced by the innate
immune system (eg, den-dritic cells, macrophages), the adaptive immune system
(especially TH1 cells), and
nonimmune cells (fibroblasts, smooth muscle cells). TNF exists in two
biologically active forms: soluble TNF and membrane-bound TNF. The biologic
activity of soluble and membrane-bound TNF is mediated by binding to TNF
receptors (TNFR) that are present on some cells (especially TH1 cells, innate immune cells, and fibroblasts). Binding of TNF
to TNFR initially activates components including NF-ÎşB that stimulate
transcription, growth, and expansion. Biologic actions ascribed to TNFR
activation include release of proinflammatory cytokines from macrophages,
T-cell activation and proliferation, fibroblast collagen production,
up-regulation of endothelial adhesion mole-cules responsible for leukocyte
migration, and stimulation of hepatic acute phase reactants. Activation of TNFR
may later lead to apoptosis (programmed cell death) of activated cells.
Three monoclonal
antibodies to human TNF are approved for the treatment of inflammatory bowel
disease: infliximab, adali-mumab, and certolizumab (Table 62–3). Infliximab and
adali-mumab are antibodies of the IgG1 subclass. Certolizumab is a recombinant
antibody that contains an Fab fragment that is conju-gated to polyethylene
glycol (PEG) but lacks an Fc portion. The Fab portions of infliximab and
certolizumab are chimeric mouse-human antibodies but adalimumab is fully
humanized. Infliximab
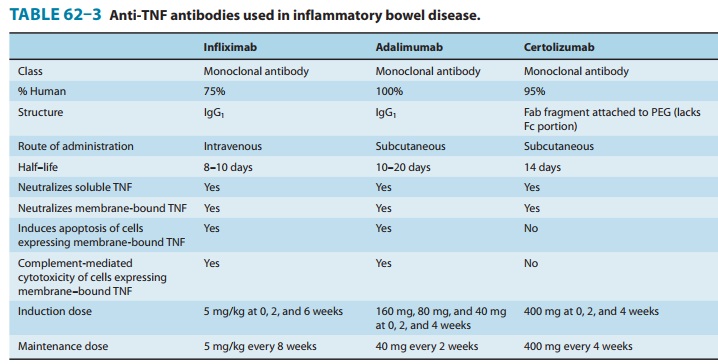
is administered as an
intravenous infusion. At therapeutic doses of 5–10 mg/kg, the half-life of
infliximab is approximately 8–10 days, resulting in plasma disappearance of
antibodies over 8–12 weeks. Adalimumab and certolizumab are administered by
subcutaneous injection. The half-life for both is approximately 2 weeks.
All three agents bind
to soluble and membrane-bound TNF with high affinity, preventing the cytokine
from binding to its receptors. Binding of all three antibodies to
membrane-bound TNF also causes reverse signaling that suppresses cytokine
release. When infliximab or adalimumab bind to membrane-bound TNF, the Fc
portion of the human IgG1 region promotes antibody-mediated apoptosis, complement
activation, and cellular cytotox-icity of activated T lymphocytes and
macrophages. Certolizumab, without an Fc portion, lacks these properties.
Clinical Uses
All three agents are
approved for the acute and chronic treatment of patients with moderate to
severe Crohn’s disease who have had an inadequate response to conventional
therapies. Infliximab also is approved for the acute and chronic treatment of
moderate to severe ulcerative colitis. With induction therapy, all three agents
lead to symptomatic improvement in 60% and disease remission in 30% of patients
with moderate to severe Crohn’s disease, including patients who have been
dependent on glucocorticoids or who have not responded to 6-MP or methotrexate.
The median time to clinical response is 2 weeks. Induction therapy is generally
given as follows: infliximab 5 mg/kg intravenous infusion at 0, 2, and 6 weeks;
adalimumab 160 mg (in divided doses) initially and 80 mg subcutaneous injection
at 2 weeks; and certolizumab 400 mg subcutaneous injection at 0, 2, and 4
weeks. Patients whorespond may be treated with chronic maintenance therapy, as
fol-lows: infliximab 5 mg/kg intravenous infusion every 8 weeks; adalimumab 40
mg subcutaneous injection every 2 weeks; certoli-zumab 400 mg subcutaneous
injection every 4 weeks. With chronic, regularly scheduled therapy, clinical response
is main-tained in more than 60% of patients and disease remission in 40%.
However, one-third of patients eventually lose response despite higher doses or
more frequent injections. Loss of response in many patients may be due to the
development of antibodies to the TNF antibody or to other mechanisms.
Infliximab is approved
for the treatment of patients with mod-erate to severe ulcerative colitis who
have had inadequate response to mesalamine or corticosteroids. After induction
therapy of 5–10 mg/wk at 0, 2, and 6 weeks, 70% of patients have a clinical
response and one third achieve a clinical remission. With contin-ued
maintenance infusions every 8 weeks, approximately 50% of patients have
continued clinical response.
Adverse Effects
Serious
adverse events occur in up to 6% of patients with anti-TNF therapy. The most
important adverse effect of these drugs is infection due to suppression of the
TH1
inflammatory response. This may lead to serious infections such as bacterial
sepsis, tuber-culosis, invasive fungal organisms, reactivation of hepatitis B,
listeriosis, and other opportunistic infections. Reactivation of latent
tuberculosis, with dissemination, has occurred. Before administering anti-TNF
therapy, all patients must undergo testing with tuberculin skin tests or
interferon gamma release assays. Prophylactic therapy for tuberculosis is
warranted for patients with positive test results before beginning anti-TNF
therapy.More common but usually less serious infections include upper
respiratory infections (sinusitis, bronchitis, and pneumonia) and cellulitis.
The risk of serious infections is increased markedly in patients taking
concomitant corticosteroids.
Antibodies
to the antibody (ATA) may develop with all three agents. These antibodies may attenuate
or eliminate the clinical response and increase the likelihood of developing
acute or delayed infusion or injection reactions. Antibody formation is much
more likely in patients given episodic anti-TNF therapy than regular scheduled
injections. In patients on chronic mainte-nance therapy, the prevalence of ATA
with infliximab is 10%, certolizumab 8%, and adalimumab 3%. Antibody
development also is less likely in patients who receive concomitant therapy
with immunomodulators (ie, 6-MP or methotrexate). Concomitant treatment with
anti-TNF agents and immunomodulators may increase the risk of lymphoma.
Infliximab intravenous
infusions result in acute adverse infusion reactions in up to 10% of patients,
but discontinuation of the infu-sion for severe reactions is required in less
than 2%. Infusion reac-tions are more common with the second or subsequent
infusions than with the first. Early mild reactions include fever, headache,
dizziness, urticaria, or mild cardiopulmonary symptoms that include chest pain,
dyspnea, or hemodynamic instability. Reactions to subsequent infusions may be
reduced with prophylactic admin-istration of acetaminophen, diphenhydramine, or
corticosteroids. Severe acute reactions include significant hypotension,
shortness of breath, muscle spasms, and chest discomfort; such reactions may
require treatment with oxygen, epinephrine, and corticosteroids.
A delayed serum
sickness-like reaction may occur 1–2 weeks after anti-TNF therapy in 1% of
patients. These reactions consist of myalgia, arthralgia, jaw tightness, fever,
rash, urticaria, and edema and usually require discontinuation of that agent.
Positive antinuclear antibodies and anti-double-stranded DNA develop in a small
number of patients. Development of a lupus-like syn-drome is rare and resolves
after discontinuation of the drug.
Rare
but serious adverse effects of all anti-TNF agents also include severe hepatic
reactions leading to acute hepatic failure, demyelinating disorders,
hematologic reactions, and new or wors-ened congestive heart failure in
patients with underlying heart disease. Anti-TNF agents may cause a variety of
psoriatic skin rashes, which usually resolve after drug discontinuation.
Lymphoma
appears to be increased in patients with untreated inflammatory bowel disease.
Anti-TNF agents may further increase the risk of lymphoma in this population,
although the relative risk is uncertain. An increased number of cases of
hepato-splenic T-cell lymphoma, a rare but usually fatal disease, have been
noted in children and young adults, virtually all of whom have been on combined
therapy with immunomodulators, anti-TNF agents, or corticosteroids.
Related Topics