Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : A brief pharmacology related to anesthesia
Intravenous anesthetics
Intravenous anesthetics (Table 12.4)
Barbiturates
The
drugs with the longest history of intravenous use in anesthesia are the
barbiturates. While many different barbiturates have been synthesized and
used, the drugs most commonly found in current anesthesia practice are
thiopental (Pen-tothal®) and methohexital (Brevital®). These drugs share the
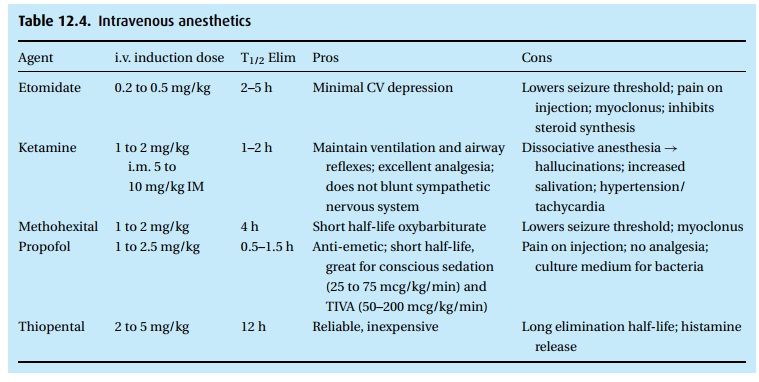
basic barbituric acid foundation (Fig. 12.1),
which by itself has no CNS depressant effect. Sub-stitutions on position 5 give
us pentobarbital, a slow- and long-acting hypnotic. Simply substituting sulfur
for the oxygen on position 2 turns the drug into the highly lipid soluble,
fast-acting thiopental.
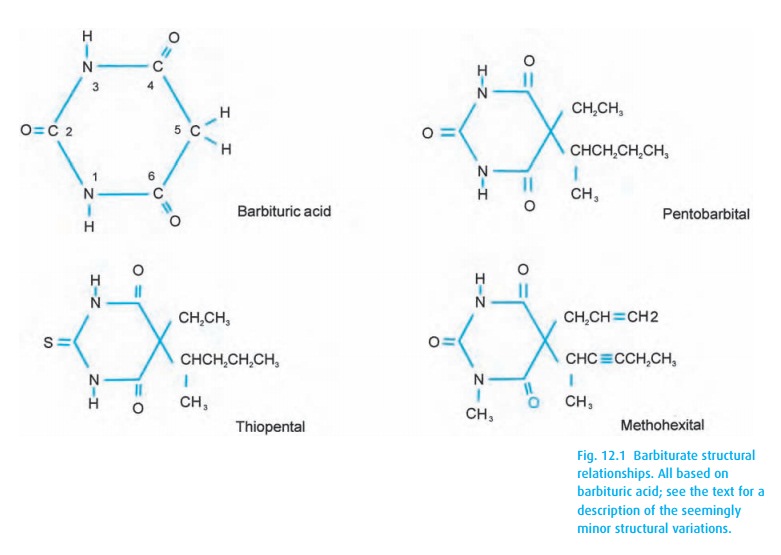
After an
intravenous thiopental bolus, e.g., 4 mg/kg, the patient falls asleep in less
than a minute and comes around again within a few more minutes. The drug
For an animated mental model of this type of drug distribution, see a simulation for a muscle relaxant; the depicted principle applies to other drugs (www.anest.ufl.edu/EA). You will appreciate that much drug not contributing to the primary and desired drug effect lingers in other body compartments. The drug in these “silent” compartments will trickle back into the circulation and the brain for a hang-over effect. Eventually, all of the drug will undergo biotransformation and excretion, but the elimination half-life of thiopental takes about 12 hours. Some of the biotransformation will convert the drug back into an oxybarbiturate (O= instead of S= in position 2), reducing lipid solubility and extending the depressant effect.
Drugs other than thiobarbiturates can have a rapid onset, as exemplified by methohexital, which is a little more potent and more rapidly metabolized than thiopental. All barbiturates used in anesthesia reduce sympathetic control of the peripheral vasculature, thereby increasing the capacitance of the venous system, which in turn leads to reduced preload. Coupled with a negative inotropic effect on the myocardium, blood pressure falls. In compensation, the baroreceptors will accelerate heart rate and mitigate the reduction of cardiac output. Respiration is also depressed. These negative effects do not play a major role in anesthesia of healthy patients where hydration with intravenous fluids can compensate for the reduced preload from venous pooling, and where we routinely overcome depressed ventilation by manual or mechanical ventilation. In addition, the barbiturates tend to constrict the blood vessels in the brain, a welcome side effect when increased intracranial pressure concerns us in patients with tumors or head trauma.
The following non-barbiturates also possess anticonvulsant effects, even in relatively small dosages.
Propofol (Diprivan®)
Propofol
is another frequently used intravenous anesthetic. As a phenol, it belongs to
an entirely different category of drugs. Not being water soluble, it is
presented in a milky-white emulsion of oil, glycerol, and lecithin (“Milk of
Amnesia”), an ideal culture medium for bacteria. Sterile technique, of course,
is mandatory for all intravenous injections. However, with propofol, we dare
not keep an open vial for later use. Because an injection of propofol into a
small vein smarts, we often elevate the arm during injection and give it
together with a local anesthetic, systemic narcotics, or a tiny dose of
thiopental or ketamine. A typical induction dose might be 1 to 2.5 mg/kg.
Propofol, 20 to 200 mcg/kg/min, is often given as a continuous infusion for
sedation or sleep, for example when a child must hold still for radiation
treatment. Together with nitrous oxide (nitrous oxide provides the analgesia,
propofol the sleep), it also serves well for short surgical procedures because
patients awaken rapidly from this technique and rarely suffer nausea or
vomiting.
Propofol
also lowers the blood pressure by a negative inotropic myocardial effect,
vasodilatation, venous pooling, and reduced preload. It depresses ventila-tion
and reduces cerebral perfusion. Occasionally, patients show mild myoclonic
movements during propofol infusion. The drug is cleared from the plasma much
more rapidly than thiopental, and the lack of a hangover and freedom from
nausea after recovery have secured propofol a place in outpatient anesthesia.
Etomidate (Amidate®)
This
drug is chemically unrelated to barbiturates and propofol and enjoys the
(per-haps overrated) reputation of causing little cardiovascular depression. It
finds use primarily in patients with heart disease where 0.2 to 0.5 mg/kg is
given for induc-tion of anesthesia – preferably into a large vein with a
rapidly running infusion in order to minimize pain from venous irritation.
Etomidate lowers the seizure threshold. In up to half of all patients, it
triggers a myoclonus, which appears to be harmless. The drug inhibits cortisol
and aldosterone synthesis (via dose-dependent inhibition of 11 β-hydroxylase),
a feature that makes it unsuitable for long-term intravenous sedation in the
ICU.
Ketamine (Ketalar®)
This
drug occupies a peculiar position between the induction agents on the one hand
and the narcotic analgesics on the other. It provides both sleep and anal-gesia
– but at a cost. The drug is the grandchild of phencyclidine, a nasty com-pound
that made a brief appearance as an intravenous anesthetic, soon aban-doned, and
now mainly encountered as a psychedelic drug on the street and known under a
medley of colorful names (PCP, Angel Dust, Dust, Sherm, Super Weed, Killer Weed,
Elephant, Embalming Fluid, Hog, PCE, Rocket Fuel, TCP). Ketamine has shed
almost all of the psychedelic effects of phencyclidine. It pro-vides excellent
analgesia (via stimulation of both NMDA2
and opioid receptors), particularly of the integument and less so of the
intestinal tract. It is classified as a dissociative anesthetic because, in low
doses, the patient may appear to be awake (perhaps with open eyes and
nystagmus) but unresponsive to sensory input. Only in deep anesthesia does the
drug obtund airway reflexes, while in light anesthesia with spontaneous
ventilation, bronchodilation is a welcome side effect, although increased
secretions are bothersome. Unlike the other common anesthetic agents, ketamine stimulates the sympathetic nervous
system, tending to increase heart rate and blood pressure. As such, it is often
the drug of choice in the hypovolemic trauma patient. However, in cardiac
patients in congestive fail-ure whose sympathetic system may be exhausted, the
drug can reveal its direct myocardial depressant effect. Ketamine would also be
relatively contra-indicated for patients at risk of high intracranial pressure,
as its sympathetic stimulation increases cerebral blood flow. On awakening from
the effect of the drug, many adults, but usually no pediatric patients,
experience visual hallucinations and delirium.
In
increasing dosages, we use ketamine for analgesia (0.2–0.5 mg/kg i.v.) or for
induction of anesthesia (1 to 2 mg/kg i.v. or intramuscularly 5–10 mg/kg). To
minimize the frequency of delirium, we give it together with one of the
benzodia-zepines and to decrease secretions, we add an anti-sialogogue.
Related Topics