Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : A brief pharmacology related to anesthesia
Inhalation anesthetics
Inhalation anesthetics (Table 12.5)
Before
discussing the agents one by one, we need to deal with the question of the
uptake and distribution of inhaled drugs.
Uptake and distribution of inhaled anesthetics
Behind this bland title lurks a concept that has baffled students
for years, yet it is fairly straightforward. Here are the facts:
(i) Solubility of the anesthetic in blood has
nothing to do with its potency. Indeed, anesthetic effectiveness has to do with
the partial pressure of the drug and not with the amount of drug in solution.
(ii) Anesthetics taken up by the blood flowing
through the lungs are distributed into different body compartments, depending
on the blood flow these com-partments receive, the volume of the compartment,
and the solubility of the anesthetic agent in that compartment.
(iii) The partial pressure exerted by a vapor in
solution has nothing to do with the ambient pressure, but has much to do with
the temperature of the solution.
Let us take these three items one by one:
(i) Solubility of the anesthetic in blood has nothing to do with its potency. Table 12.5tells the story. At equilibrium, you will find 12 times as much ether (when we say “ether” we refer to diethyl ether; some of the halogenated anesthetics are chemically also ethers, but we call them by their given name, e.g., sevoflurane and desflurane) in blood than in the overlying gas (blood/gas partition coefficient). The blood practically slurps up the ether. Every breath that brings in more ether dumps its load of the anesthetic into the blood perfusing the lungs. It takes breath after breath to deliver enough ether for the blood to come into equilibrium with the alveolar gas. At equilibrium, the partial pressure (but not the concentration per unit of volume) of the ether in the gas phase (alveolar gas) is the same as in the blood.
We have
picked ether (no longer used in the Western world but widely used elsewhere)
because of its extraordinary solubility in blood at body tempera-ture. In
comparisson, look at sevoflurane. Ether is 20 times as soluble in blood as is
sevoflurane. We can quickly bring enough sevoflurane into the alveoli to
establish an equilibrium between alveolar gas and blood. For ether, it will
take many, many breaths laden with ether to fill the blood compartment and to
reach equilibrium between alveolar gas and blood. Yet, diethyl ether and
sevoflurane have almost identical MAC values. MAC stands (neither for a
computer nor for a truck) for minimal alveolar concentration, namely the
concentration in alveolar gas at which 50% of patients no longer respond to a
painful stimulus. Thus, when we have attained MAC values for ether and MAC
values for sevoflurane, there will be much, much more ether dissolved in the
patient than will be true for sevoflurane. It will be quicker to get the
patient to sleep – and have him wake up again – with sevoflurane than with
ether.
Observe
in Table 12.5that, at equilibrium, you will
find five times as much ether in fat than in blood and 45 times as much
isoflurane in fat than in blood . . . which brings us to the next point.
(ii) Anesthetics taken up by the lungs are distributed into
different body com-partments, depending on the blood flow these compartments
receive, their volume, and the solubility of the anesthetic agent in that
compartment.
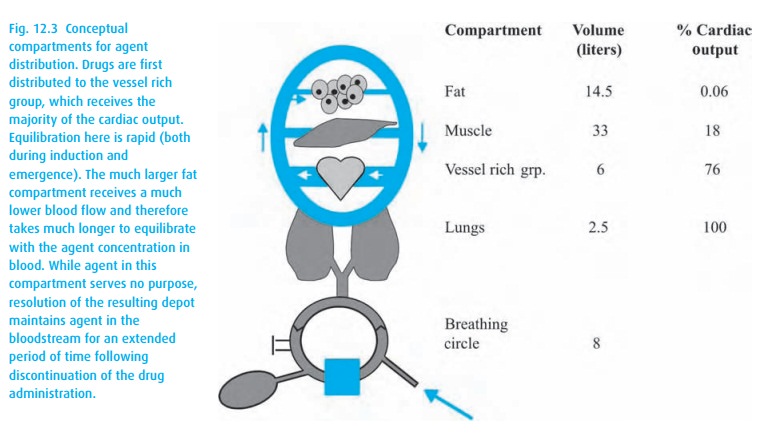
Figure 12.3shows the relationships. Observe the low blood
flow and large volume of the fat compartment (not even assuming an obese
patient!) and
These
“compartments” are conceptual rather than anatomical; the vessel rich group
contains heart and brain as well as kidney and liver.
You can
easily imagine that during a long anesthetic, the fat compartment, despite its
low perfusion, will accumulate much anesthetic agent because inhalation
anesthetics are so very soluble in fat (they make excellent grease stain
removers). At the end of the anesthetic, the poorly perfused fat compart-ment
will slowly deliver anesthetic to the venous blood, causing the patient to have
a protracted recovery from the anesthetic; the greater the solubility of the
agent in fat, the more protracted.
(iii) The partial pressure exerted by a vapor in
solution has nothing to do with the ambient pressure, but has much to do with
the temperature of the solution.
Water
vapor in the lungs at 37 °C has a vapor pressure of 47 mmHg. At that
temperature, as many molecules of water leave the blood as enter it. The vapor
pressure increases with rising temperatures. At the boiling point, the vapor
pressure equals ambient pressure (at the top of the mountain you need to boil
your egg a little longer because the water will boil at a lower temper-ature).
At sea level (1 atmosphere or 760 mmHg ambient pressure), it takes 1.15% of
isoflurane to render 50% of the population unresponsive to noxious (if the
patient were awake the word would be “painful”) stimuli. At that baro-metric
pressure, 1.15% equals about 9 mmHg. At altitude with a barometric pressure of
500 mmHg, these same 9 mmHg would be about 1.8% of vapor in the alveolar gas.
Thus, the convention of reporting anesthetic concentrations in percent – as our
vaporizers do – leaves something to be desired. In Table 12.6, we compare isoflurane
MAC values in two cities of very different alti-tude that happen to have the
same name. Remember that about half of our patients will be responsive, i.e.,
with a movement without being necessarily conscious, at 1 MAC. In order to have
almost 100% of patients unrespon-sive to noxious (painful) stimuli, we need to
expose them to 1.3 MAC. Also, remember that most patients have been given other
CNS depressants; MAC values change with age (down they go); and distribution of
the anesthetic agents also depends on the patient’s cardiac output, which, in
shock with a very low cardiac output, may send a disproportionate percentage of
the blood to brain and heart.
We can
anticipate that many CNS depressants will lower MAC. Intuitively not so
obvious, however, are reports that hyponatremia, metabolic acidosis, alpha
methyldopa, chronic dextroamphetamine usage, levodopa, and alpha-2 agonists can
lower MAC, as does pregnancy. We find elevated MAC values in hyperna-tremia,
hyperthermia, and in patients taking monoamine oxidase inhibitors, cocaine and
ephedrine. The administration of a sympathomimetic can some-times lighten
anesthesia. Because we always titrate anesthetics to a desired effect and
because patients vary greatly in their response to drugs – anesthetics as well
as others – these differences in MAC rarely influence our anesthetic practice.
The gases
Only two
anesthetic gases (as opposed to vapors) deserve to be mentioned: nitrous oxide
and xenon. Cyclopropane and ethylene are two explosive gases used in the past.
Nitrous oxide
Nitrous
oxide has been around for centuries and is still widely used. Yet you will
often hear it said that, if nitrous oxide were to be introduced today, it would
never pass the FDA’s muster. For this jaundiced view, we can cite several
reasons.
·
The gas is a weak anesthetic with a MAC of 105%. Thus, it would
require a hyperbaric chamber to administer that concentration with enough
oxygen to make it safe. In concentrations up to 70% in oxygen, it is an
analgesic rather than a reliable anesthetic.
·
Because it is such a weak drug, in the past people tended to give
high concen-trations of it, which is another way of saying that it was given
with marginal concentrations of oxygen. Modern anesthesia machines will not let
you give less than 25% oxygen, but many patients with ventilation/perfusion
abnor-malities require a higher FiO2.
·
It does some peculiar things to some important enzymes. By
oxidizing vitamin-B12-dependent enzymes (methionine and thymidylate
synthetase), it inhibits formation of myelin and thymidine (important in DNA
synthesis). Prolonged exposure to nitrous oxide has caused neuropathy and
megaloblas-tic changes as well as leukopenia. A decreased white count was
noticed in tetanus patients requiring prolonged mechanical ventilation during
which nitrous oxide was used continuously as an analgesic sedative. Attempting
to use this effect to advantage, subsequent experiments with nitrous oxide in
leukemic patients confirmed the observation that the gas could reduce the white
count. Unfortunately, the effect did not last and upon discontinu-ation of the
gas, the cell counts rose back to their pathologic condition. The neuropathic
effect of nitrous oxide was observed by a neurologist who saw dentists
complaining of different degrees of apraxia, ataxia, and impotence. Exposure to
nitrous oxide was the common denominator in these patients. These effects are
not observed during the relatively brief use (minutes or hours instead of
repeated use or days of exposure) of nitrous oxide in patients undergoing
surgical anesthesia.
·
Despite its low blood solubility (blood/gas partition coefficient
of 0.47), the high concentration of N2O administered (50% to 70% in
oxygen) causes many liters to dissolve in the body during a lengthy anesthetic.
Because it diffuses readily into air-containing bubbles, nitrous oxide can
increase the volume of air in the cuff of an endotracheal tube, the gas in the
bowel, a bleb in the lung, or gas in the middle ear. The volume of a closed air
space, e.g., pneumothorax, will double in just 10 minutes! The doubling time
for bowel is much slower (hours).
·
We might also mention that it supports combustion, almost as well
as oxygen.
·
For neurosurgical procedures, even low-dose and brief exposure to
nitrous oxide affects evoked potentials – which we monitor to keep an eye on
the integrity of the spinal cord, among other things.
·
Based on questionable epidemiologic data and on animal experiments,
nitrous oxide has been accused of causing spontaneous abortion in person-nel
repeatedly exposed to trace concentrations of the gas. Consequently, maximal
acceptable trace concentrations of nitrous oxide in the OR have been
established by the government: OSHA calls for a time weighted aver-age
concentration of less than 25 parts per million.
·
Finally, thrill seekers have extensively abused nitrous oxide,
obtaining it legally (and stupidly) in the small whippet cylinders. There, the
gas exists in its pure form, that is without oxygen. The ill-informed who
inhale it from such a source expose themselves to the double trouble of
inhaling a hypoxic gas mixture while breathing a harmful gas.
In
fairness, we have to say something positive about the gas. Because of its low
solubility, it does not take much time to reach equilibrium between alveolar
gas and blood, which translates into fairly rapid induction and emergence with
min-imal cardiovascular side effects. Some pediatric dentists like its mild
analgesic effect and the fact that it is tasteless and odorless (which is why
industry uses it as a propellant for canned whipped cream). In the pediatric
dental practice, nitrous oxide is usually administered in concentrations
between 30% and 50% in oxygen. Higher concentrations of nitrous oxide given by
itself often lead to excite-ment. In anesthetic practice, therefore, we
administer the gas together with other CNS depressants, for example thiopental
or propofol or a halogenated anesthetic vapor.
Even
though it has nothing to do with the pharmacology of nitrous oxide, and
everything to do with the fact that we give it in high concentrations (up to
70% – whereas the halogenated agents are given in less than 1/10th that
concentration), we need to mention three concepts linked to nitrous oxide: the
second gas effect; the augmented inflow effect (also called the concentration
effect); and diffusion hypoxia.
The second gas effect
If you
administer a high concentration of nitrous oxide to the lungs during induc-tion
of anesthesia, much of the gas will go into solution in the body, thereby
reducing its partial pressure in the lungs. The sum of all partial pressures
will equal barometric pressure. In other words, if a large volume of nitrous
oxide van-ishes, any other (second) (anesthetic) gas present in the lung will
experience an increase in its partial pressure, which will speed its uptake by
the blood.
The augmented inflow or concentration effect
Because
of the large uptake of nitrous oxide, the exhaled volume will be dimin-ished,
enabling the next breath to have an increased tidal volume to re-establish
normal lung volume.
Diffusion hypoxia
After
hours of anesthesia with nitrous oxide, many liters of the gas go into solution
in the body. At the end of anesthesia, when the patient no longer inhales
nitrous oxide, the liters of nitrous oxide in solution will follow their
concentration gradient and be delivered to the lung where the gas will displace
other gases – including oxygen. Thus, we give oxygen for a few breaths at the
end of anesthesia and thus prevent diffusion hypoxia.
Xenon
This
noble gas is even less soluble than nitrous oxide (blood/gas partition
coeffi-cient of 0.12) and about twice as potent (MAC = 56%). In addition, it appears to have no major depressant effects
on the cardiovascular system. We do not know how it produces anesthesia, being
a noble gas (we don’t really know how the other not so noble agents do it,
either). Xenon would make a desirable anesthetic, were it not for its high cost
(about $17/L). Xenon is currently not used in the USA and most studies of the
gas come from abroad.
The anesthetic vapors
Ethers
Anesthetic
vapors exist as fluids at ambient conditions. They have low vapor pressures,
and the vapors overlying the liquid phase have anesthetic properties. It all
started with diethyl ether, the granddaddy of anesthetic vapors. Over the last
150 years, uncounted chemists have rearranged the structure of these substances
and, by adding halogens, have developed a host of promising anesthetics. Each
has distinctive vapor pressures, blood/gas partition coefficients, potencies
(see Table 12.5), and side effects, e.g.,
upper airway irritation, bronchodilation, cardiac irritability.
With the
arrival of the non-flammable agents, i.e., halothane (Fluothane®) and the
halogenated ethers, we were able to retire from clinical use the highly
flammable diethyl ether. Methoxyflurane (Metofane®) was abandoned because of
its extensive biotransformation, which led to the liberation of enough fluoride
ions to damage the kidneys, causing a vasopressin-resistant high output renal
failure. The much less extensive biotransformation of enflurane (Ethrane®) and
sevoflurane (Ultane®) also liberates fluoride ions but in such small
concentra-tions that renal problems have not been a cause for concern. Initial
worries over nephrotoxicity from sevoflurane’s degradation by CO2
absorbent in the anes-thesia circuit (forming the dreaded “Compound A,” also
known as pentafluor-isoprenyl fluoromethyl) appears to lack clinical relevance
(unless anesthetizing a rat).
Halogenated aliphatic compounds
So much
for the halogenated ethers. Now to a different class, the halogenated aliphatic
compounds, the ancestor of which, chloroform (HCCl3), dates back to
1847 when it was first shown to be an anesthetic. While neither irritating nor
combustible (a big problem for diethyl ether), it eventually fell out of favor
because of its propensity to cause arrhythmias and hepatic damage. A number of
other halogenated aliphatic compounds came and went, until finally in the mid
1960s, halothane appeared and was soon widely used. It is still around, even
though it had its lumps and bumps. It sensitizes the heart to arrhythmias
triggered by catecholamines.
Halothane hepatitis
Soon
after the introduction of halothane worrisome reports of “halothane hepati-tis”
appeared. Fever, malaise, and evidence of liver damage as seen in the elevation
of serum aminotransferases pointed to liver damage. Not the halothane molecule
itself but the products of its biotransformation cause the trouble. Halothane
falls prey to a reductive and an oxidative breakup, the former exaggerated in
the pres-ence of hypoxemia, the latter in some patients causing an immune response
that can set the stage for severe halothane hepatitis at a future exposure to
halothane. Hepatitis after halothane anesthesia is rare (perhaps 1 in 30 000)
and much rarer after the other halogenated anesthetics. The extent of
biotransformation of the drug might play a role: halothane stands out with 20%
to 46% of the agent under-going biotransformation as compared to isoflurane
(0.2% to 2%) and desflurane (0.02%). The products of biotransformation of
sevoflurane (2% to 5% metabo-lized) appear to cause no harm to the liver.
Comparing effects on heart, lung, and brain
All
anesthetic vapors affect consciousness and have analgesic effects. They depress
ventilation, as judged by decreasing minute ventilation and increasing levels
of arterial carbon dioxide, with increasing depth of anesthesia. A few words
about generally subtle differences between these drugs:
Inhalation induction
The
older halothane and the newer sevoflurane have established for themselves a
special niche because they are less irritating to the upper airway than the
others. Particularly in children, who abhor needle sticks (and whose veins are
more easily cannulated when the child is asleep), anesthesia can be induced
quite gently by inhalation of nitrous oxide/oxygen together with either one of
these two drugs.
Cardiovascular effects
All
volatile agents depress myocardial contractility and cause peripheral
vasodi-latation. As long as baroreceptors function normally, heart rate will
increase in response to hypotension. In deep anesthesia, this compensation will
not suffice to prevent a drop in cardiac output. Here, halothane occupies an
unusual position. It inhibits the baroreceptor; consequently, we see less
tachycardia (even bradycar-dia in deeply anesthetized children) during
halothane-induced hypotension and a greater drop in cardiac output than is true
for the other agents at comparable levels of anesthesia. Another oddity
regarding halothane anesthesia: otherwise well-tolerated levels of circulating
catecholamines, whether injected or liberated by the body, trigger arrhythmias
in the presence of halothane.
Respiratory effects
Under
very deep anesthesia, ventilation stops, usually before the heart arrests.
Thus, a respiratory arrest from an overdose with an inhalation anesthetic need
not be fatal if discovered in time, and if ventilation of the (still perfused)
lungs with oxygen can remove the volatile anesthetic.
In
surgical anesthesia, spontaneous ventilation will still be maintained IF the
patient was not given other drugs that depress ventilation – such as opiates –
and IF the patient is not paralyzed by neuromuscular blocking drugs, so
commonly used in order to relax striated muscles and thus ease the surgeon’s
job.
In general, all halogenated inhalation anesthetics decrease minute ventilation by decreasing tidal volume. The compensatory increase in respiratory rate cannot prevent a respiratory acidosis (and hypoxemia when breathing room air) because any increase in respiratory rate increases the ventilation of dead space. Respir-atory depression and tachypnea are less pronounced with desflurane (Suprane®) and sevoflurane than with halothane, with isoflurane (Forane®) lying somewhere in between.
Under
inhalation anesthesia, patients respond only sluggishly to rising arterial
carbon dioxide levels (= respiratory depression). Even low
concentrations of the inhalation agents also depress the chemoreceptor response
to hypoxemia.
Central nervous system effects
The
inhalation anesthetics depress, in a dose-dependent manner, CNS function – as
shown by clinical findings starting with a state of somnolence, during which
the patient can still respond – to coma, in which external noxious (we do not
call it “painful” as you have to be conscious to find something painful!)
stimulation elicits no visible response. This sentence was carefully chosen,
because invisible CNS responses are detectable by electroencephalography and
evoked potentials; these persist long after motor responses have been
abolished. Eventually, they too vanish in deep anesthesia. Halogenated
inhalation agents tend to increase cerebral blood flow, which is not a
desirable effect in patients at risk of brain swelling. In neurosurgical
anesthesia, we rely greatly on intravenous techniques using the inhalation
agents only in low doses and as adjuncts.
Related Topics