Chapter: Medical Surgical Nursing: Management of Patients With Neurologic Dysfunction
Increased Intracranial Pressure
Increased Intracranial
Pressure
The
rigid cranial vault contains brain tissue (1,400 g), blood (75 mL), and CSF (75
mL) (Hickey, 2003). The volume and pressure of these three components are usually
in a state of equilibrium and produce the ICP. ICP is usually measured in the
lat-eral ventricles; normal ICP is 10 to 20 mm Hg (Hickey, 2003).
The
Monro-Kellie hypothesis states that
because of the lim-ited space for expansion within the skull, an increase in
any one of the components causes a change in the volume of the others. Because
brain tissue has limited space to change, compensation typically is
accomplished by displacing or shifting CSF, increas-ing the absorption of CSF,
or decreasing cerebral blood volume. Without such changes, ICP will begin to
rise. Under normal cir-cumstances, minor changes in blood volume and CSF volume
occur constantly due to alterations in intrathoracic pressure (coughing,
sneezing, straining), posture, blood pressure, and sys-temic oxygen and carbon
dioxide levels.
Pathophysiology
Increased ICP is a syndrome that affects many
patients with acute neurologic conditions. This is because pathologic
conditions alter the relationship between intracranial volume and pressure.
Although an elevated ICP is most commonly associated with head injury, it also
may be seen as a secondary effect in other condi-tions, such as brain tumors,
subarachnoid hemorrhage, and toxic and viral encephalopathies. Increased ICP
from any cause de-creases cerebral perfusion, stimulates further swelling
(edema), and shifts brain tissue through openings in the rigid dura, result-ing
in herniation, a dire, frequently
fatal event.
DECREASED CEREBRAL BLOOD FLOW
Increased
ICP may significantly reduce cerebral blood flow, re-sulting in ischemia and
cell death. In the early stages of cerebral ischemia, the vasomotor centers are
stimulated and the systemic pressure rises to maintain cerebral blood flow.
Usually a slow bounding pulse and respiratory irregularities accompany this.
These changes in blood pressure, pulse, and respiration are im-portant
clinically because they suggest increased ICP.
The
concentration of carbon dioxide in the blood and in the brain tissue also has a
role in the regulation of cerebral blood flow. A rise in carbon dioxide partial
pressure (PaCO2) causes cerebral
vasodilatation, leading to increased cerebral blood flow and in-creased ICP; a
fall in PaCO2 has a vasoconstrictive effect
(Young, Ropper & Bolton, 1998). Decreased venous outflow may also in-crease
cerebral blood volume, thus raising ICP.
CEREBRAL EDEMA
Cerebral edema or swelling is defined as an
abnormal accumula-tion of water or fluid in the intracellular space,
extracellular space,or both, associated with an increase in brain tissue
volume. Edema can occur in the gray, white, or interstitial matter. As brain
tissue swells within the rigid skull, several mechanisms at-tempt to compensate
for the increasing ICP. These mechanisms include autoregulation and decreasing
the production and flow of CSF. Autoregulation
refers to the brain’s ability to change the diameter of its blood vessels
automatically to maintain a constant cerebral blood flow during alterations in
systemic blood pressure.
CEREBRAL RESPONSE TO INCREASED ICP
As ICP rises, compensatory mechanisms in the brain
work to maintain blood flow and prevent tissue damage. The brain can maintain a
steady perfusion pressure when the arterial systolic blood pressure is 50 to
150 mm Hg and ICP is less than 40 mm Hg. The cerebral perfusion pressure is
calculated by subtracting the ICP from the mean arterial pressure. For example,
if the mean arterial pressure is 100 and the ICP is 15, then the cerebral
per-fusion pressure is 85 mm Hg. The normal cerebral perfusion pressure is 70 to
100 mm Hg (Hickey, 2003; Young et al., 1998). As ICP rises, however, and the
autoregulatory mechanism of the brain is overwhelmed, cerebral perfusion
pressure can rise to greater than 100 mm Hg or fall to less than 50 mm Hg.
Patients with a cerebral perfusion pressure less than 50 mm Hg experience
irreversible neurologic damage. If ICP equals mean arterial pres-sure, cerebral
circulation ceases (Porth, 2002).
A clinical phenomenon known as the Cushing’s response (or Cushing’s
reflex) is seen when cerebral blood flow decreases significantly. When
ischemic, the vasomotor center triggers a rise in arterial pressure in an
effort to overcome the increased ICP. A sympathetically mediated response
causes a rise in the systolic blood pressure with a widening of the pulse
pressure and cardiac slowing. This response, which is mediated by the
sympathetic nervous system, is seen clinically as a rise in systolic blood
pres-sure, widening of the pulse pressure, and reflex slowing of the heart
rate. This is a sign requiring immediate intervention; how-ever, perfusion may
be recoverable if treated rapidly.
At a certain volume or pressure, the brain’s ability to auto-regulate becomes ineffective and decompensation (ischemia and infarction) begins (Young et al., 1998). When this occurs, the pa-tient exhibits significant changes in mental status and vital signs. The bradycardia, hypertension, and bradypnea associated with this deterioration are known as Cushing’s triad, a grave sign. At this point, herniation of the brain stem and occlusion of the cerebral blood flow occur if therapeutic intervention is not initiated. Her-niation refers to the shifting of brain tissue from an area of high pressure to an area of lower pressure (Fig. 61-2).
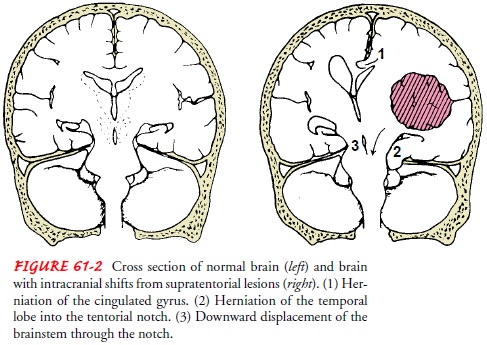
The herniated tissue
exerts pressure on the brain area to which it has herniated or shifted,
interfering with the blood supply in that area. Cessa-tion of cerebral blood
flow results in cerebral ischemia and in-farction and brain death.
Clinical Manifestations
When ICP increases to the point at which the
brain’s ability to adjust has reached its limits, neural function is impaired;
this may be manifested by clinical changes first in LOC and later by abnormal
respiratory and vasomotor responses.
Any sudden change in the patient’s condition, such
as rest-lessness (without apparent cause), confusion, or increasing
drowsi-ness, has neurologic significance. These signs may result from
compression of the brain due to swelling from hemorrhage or edema, an expanding
intracranial lesion (hematoma or tumor), or a combination of both.
As ICP increases, the patient becomes stuporous,
reacting only to loud auditory or painful stimuli. At this stage, serious
impair-ment of brain circulation is probably taking place, and immedi-ate
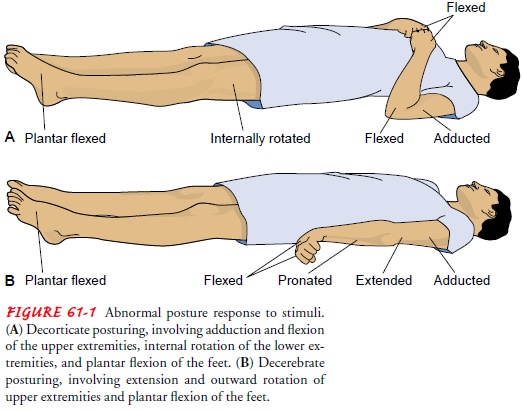
intervention is required. As neurologic function deteriorates further, the
patient becomes comatose and exhibits abnormal motor responses in the form of decortication, decerebration, or flaccidity (see Fig. 61-1). When the coma is
profound, with the pupils dilated and fixed and respirations impaired, death is
usu-ally inevitable.
Assessment and Diagnostic Findings
The diagnostic studies used to determine the
underlying cause of increased ICP. The patient may undergo cerebral
angiography, computed tomography (CT) scanning, magnetic resonance imaging
(MRI), or positron emis-sion tomography (PET). Transcranial Doppler studies
provide information about cerebral blood flow. The patient with increased ICP
may also undergo electrophysiologic monitoring to monitor cerebral blood flow
indirectly. Evoked potential monitoring mea-sures the electrical potentials
produced by nerve tissue in response to external stimulation (auditory, visual,
or sensory). Lumbar puncture is avoided in patients with increased ICP because
the sudden release of pressure can cause the brain to herniate.
Complications
Complications
of increased ICP include brain stem herniation, diabetes insipidus, and
syndrome of inappropriate antidiuretic hormone (SIADH).
Brain
stem herniation results from an excessive increase in ICP, when the pressure
builds in the cranial vault and the brain tissue presses down on the brain
stem. This increasing pressure on the brain stem results in the cessation of
blood flow to the brain, causing irreversible brain anoxia and brain death.
Diabetes
insipidus is the result of decreased secretion of anti-diuretic hormone. The
patient has excessive urine output, and hyperosmolarity results (Young et al.,
1998). Therapy consists of administration of fluid volume, electrolyte
replacement, and va-sopressin (desmopressin, DDAVP) therapy.
SIADH is the result of increased secretion of
antidiuretic hor-mone. The patient becomes volume-overloaded, urine output
di-minishes, and serum sodium concentration becomes dilute. Treatment of SIADH
includes fluid restriction, which is usually sufficient to correct the
hyponatremia; severe cases call for judi-cious administration of a 3%
hypertonic saline solution (Hickey, 2003). Patients with chronic SIADH may
respond to lithium car-bonate or demeclocycline, which reduces renal tubule
respon-siveness to antidiuretic hormone.
Management
Increased ICP is a true emergency and must be
treated promptly. Invasive monitoring of ICP is an important component of
man-agement, but immediate management to relieve increased ICP involves
decreasing cerebral edema, lowering the volume of CSF, or decreasing cerebral
blood volume while maintaining cerebral perfusion (Cunning & Houdek, 1999).
These goals are accom-plished by administering osmotic diuretics and
corticosteroids, restricting fluids, draining CSF, controlling fever,
maintaining systemic blood pressure and oxygenation, and reducing cellular
metabolic demands. Judicious use of hyperventilation is recom-mended only if
the ICP is refractory to other measures.
MONITORING ICP
The purposes of ICP monitoring are to identify
increased pressure early in its course (before cerebral damage occurs), to
quantify the degree of elevation, to initiate appropriate treatment, to provide
access to CSF for sampling and drainage, and to evaluate the ef-fectiveness of
treatment. An intraventricular catheter (ventricu-lostomy), a subarachnoid
bolt, an epidural or subdural catheter, or a fiberoptic transducer-tipped
catheter placed in the subdural space or the ventricle can be used to monitor
ICP (Fig. 61-3).
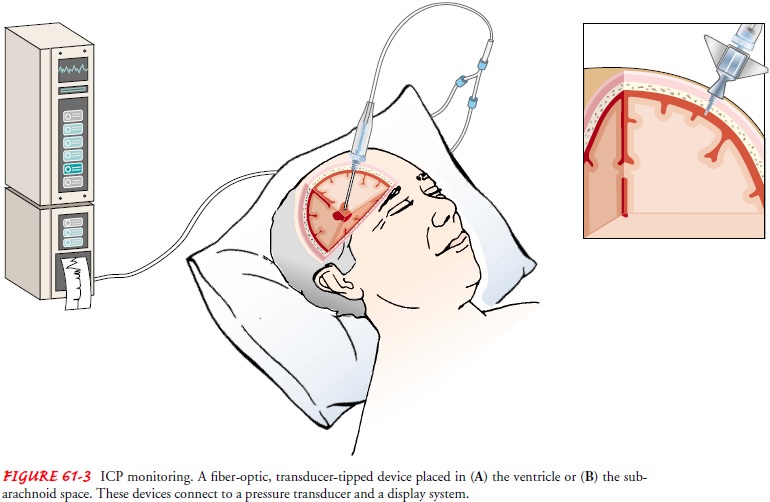
When a ventriculostomy
or ventricular catheter monitoring device is used for monitoring ICP, a
fine-bore catheter is inserted into a lateral ventricle, usually in the
nondominant hemisphere of the brain (Hickey, 2003). The catheter is connected
by a fluid-filled system to a transducer, which records the pressure in the
form of an electrical impulse. In addition to obtaining continu-ous ICP
recordings, the ventricular catheter allows CSF to drain, particularly during
acute rises in pressure. The ventriculostomy also can be used to drain the
ventricle of blood. Also, continuous drainage of ventricular fluid under
pressure control is an effective method of treating intracranial hypertension.
Another advantage of an indwelling ventricular catheter is the access it
provides for the intraventricular administration of medications and the
instil-lation of air or a contrast agent for ventriculography. Complica-tions
include ventricular infection, meningitis, ventricular collapse, occlusion of
the catheter by brain tissue or blood, and problems with the monitoring system.
The subarachnoid
bolt (or screw) is a hollow device inserted through the skull and dura
mater into the cranial subarachnoid space (Hickey, 2003). It has the advantage
of not requiring a ven-tricular puncture. The subarachnoid screw is attached to
a pres-sure transducer, and the output is recorded on an oscilloscope. The
hollow screw technique has the advantage of avoiding com-plications from brain
shift and small ventricle size. Complications include blockage of the screw by
clot or brain tissue, which leads to a loss of pressure tracing and a decrease
in accuracy at high ICP readings.
An epidural monitor uses a pneumatic flow sensor that func-tions on a nonelectrical basis. This pneumatic epidural ICP monitoring system has a low incidence of infection and
complications and appears to read pressures accurately. Calibration of the
system is maintained automatically, and abnormal pressure waves trig-ger an
alarm system. One disadvantage of the epidural catheter is the inability to
withdraw CSF for analysis.
A
fiberoptic monitor,
or transducer-tipped catheter, is be-coming a widely used alternative to
standard intraventricular, subarachnoid, and subdural systems (Hickey, 2003).
The minia-ture transducer reflects pressure changes, which are converted to
electrical signals in an amplifier and displayed on a digital moni-tor. The
catheter can be inserted into the ventricle, subarachnoid space, subdural
space, or brain parenchyma or under a bone flap. If inserted into the
ventricle, it can also be used in conjunction with a CSF drainage device.
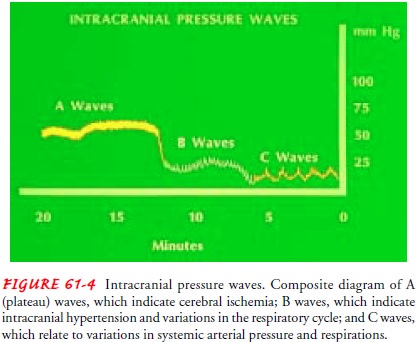
Waves of high pressure and troughs of relatively
normal pressure indicate changes in ICP. Waveforms are captured and recorded on
an oscilloscope. These waves have been classified as A waves (plateau waves), B
waves, and C waves (Fig. 61-4). The plateau waves (A waves) are transient,
paroxysmal, recurring ele-vations of ICP that may last 5 to 20 minutes and
range in ampli-tude from 50 to 100 mm Hg (Hickey, 2003). Plateau waves have
clinical significance and indicate changes in vascular volume within the
intracranial compartment that are beginning to com-promise cerebral perfusion.
A waves may increase in amplitude and frequency, reflecting cerebral ischemia
and brain damage that can occur before overt signs and symptoms of raised ICP
are seen clinically. B waves are shorter (30 seconds to 2 minutes), with
smaller amplitude (up to 50 mm Hg). They have less clinical significance, but
if seen in runs in a patient with depressed con-sciousness, they may precede
the appearance of A waves. B waves may be seen in patients with intracranial
hypertension and de-creased intracranial compliance. C waves are small,
rhythmic os-cillations with frequencies of approximately six per minute. They appear
to be related to rhythmic variations of the systemic arte-rial blood pressure
and respirations.
DECREASING CEREBRAL EDEMA
Osmotic diuretics (mannitol) may be given to dehydrate the brain tissue and reduce cerebral edema. They act by drawing water across intact membranes, thereby reducing the volume of brain and extracellular fluid. An indwelling urinary catheter is usually inserted to monitor urinary output and to manage the re-sulting diuresis. When a patient is receiving osmotic diuretics, serum osmolality should be determined to assess hydration sta-tus. Corticosteroids (eg, dexamethasone) help reduce the edema surrounding brain tumors when a brain tumor is the cause of increased ICP.
Another
method for decreasing cerebral edema is fluid restric-tion (Hickey, 2003).
Limiting overall fluid intake leads to dehy-dration and hemoconcentration,
drawing fluid across the osmotic gradient and decreasing cerebral edema.
Conversely, overhydra-tion of the patient with increased ICP is avoided, as
this will in-crease cerebral edema.
It
has been hypothesized that lowering body temperature will decrease cerebral
edema, reduce the oxygen and metabolic re-quirements of the brain, and protect
the brain from continued is-chemia. If body metabolism can be reduced by
lowering body temperature, the collateral circulation in the brain may be able
to provide an adequate blood supply to the brain. The effect of hypothermia on
ICP requires more study (Slade, Kerr & Marion, 1999), but as yet induced
hypothermia has not been proven to be beneficial in the brain-injured patient
(Clifton, Miller, Choi et al., 2001). Inducing and maintaining hypothermia is a
major clini-cal procedure and requires knowledge and skilled nursing
obser-vation and management.
MAINTAINING CEREBRAL PERFUSION
The
cardiac output may be manipulated to provide adequate perfusion to the brain.
Improvements in cardiac output are made using fluid volume and inotropic agents
such as dobutamine hydro-chloride. The effectiveness of the cardiac output is
reflected in the cerebral perfusion pressure, which is maintained at greater
than 70 mm Hg (Young et al., 1998). A lower cerebral perfusion pres-sure
indicates that the cardiac output is insufficient to maintain adequate cerebral
perfusion.
REDUCING CSF AND INTRACRANIAL BLOOD VOLUME
CSF
drainage is frequently performed because the removal of CSF with a
ventriculostomy drain may dramatically reduce ICP and restore cerebral
perfusion pressure. Caution should be used in draining CSF because excessive
drainage may result in collapse of the ventricles.
Hyperventilation,
which results in vasoconstriction, has been used for many years in patients with
increased ICP. Recent re-search has demonstrated that hyperventilation may not
be as ben-eficial as once thought (Hickey, 2003). The reduction in the PaCO2
may result in hypoxia, ischemia, and an increase in cere-bral lactate levels.
Maintaining the PaCO2 at
30 to 35 mm Hg may prove beneficial. Hyperventilation is indicated in patients
whose ICP is unresponsive to conventional therapies, but it should be used
judiciously.
CONTROLLING FEVER
Preventing
a temperature elevation is critical because fever in-creases cerebral
metabolism and the rate at which cerebral edema forms. Strategies to reduce
temperature include administration of antipyretic medications, as prescribed,
and use of a cooling blan-ket. Additional strategies for reducing fever are
included in the Nursing Process: The Patient With an Altered Level of
Consciousness section. The patient’s temperature is monitored closely, and the
patient is observed for shivering, which should be avoided because it increases
ICP (Sund-Levander & Wahren, 2000).
MAINTAINING OXYGENATION
Arterial blood gases must be monitored to ensure
that systemic oxygenation remains optimal. Hemoglobin saturation can also be
optimized to provide oxygen more efficiently at the cellular level.
REDUCING METABOLIC DEMANDS
Cellular
metabolic demands may be reduced through the ad-ministration of high doses of
barbiturates when the patient is un-responsive to conventional treatment. The
mechanism by which barbiturates decrease ICP and protect the brain is
uncertain, but the resultant comatose state is thought to reduce the meta-bolic
requirements of the brain, thus providing some protection (Greenberg, 2001).
Another
method of reducing cellular metabolic demand and improving oxygenation is the
administration of pharmacologic paralyzing agents. The patient who receives
these agents cannot move, decreasing the metabolic demands and resulting in a
de-crease in cerebral oxygen demand. Because the patient cannot re-spond or
report pain, sedation and analgesia must be provided because the paralyzing
agents do not provide either.
Patients receiving high doses of barbiturates or
pharmacologic paralyzing agents require continuous cardiac monitoring,
endo-tracheal intubation, mechanical ventilation, ICP monitoring, and arterial
pressure monitoring. Pentobarbital (Nembutal), thiopen-tal (Pentothal), and
propofol (Diprivan) are the most common agents used for high-dose barbiturate
therapy (Greenberg, 2001). Serum barbiturate levels must be monitored (Hickey,
2003).
The ability to perform serial neurologic
assessments on the pa-tient is lost with the use of barbiturates or paralyzing
agents (Greenberg, 2001). Therefore, other monitoring tools are needed to
assess the patient’s status and response to therapy. Important parameters that
must be assessed include ICP, blood pressure, heart rate, respiratory rate, and
response to ventilator therapy (eg, bucking the ventilator). The level of
pharmacologic paralysis is adjusted based on serum levels and the assessed
parameters. Po-tential complications include hypotension due to decreased
sym-pathetic tone and myocardial depression (Greenberg, 2001).
TRENDS IN NEUROLOGIC MONITORING
One
controversial trend in cerebral monitoring is the ongoing measurement of venous
oxygen saturation in the jugular bulb (SjO2).
Readings taken from a catheter residing in the jugular outflow tract
theoretically allow for a comparison of arterial and venous oxygen saturation,
and the balance of cerebral oxygen supply and demand is demonstrated. Venous
jugular desatura-tions can reflect early cerebral ischemia, alerting the
clinician prior to a rise in ICP. Minimizing elevations in ICP can poten-tially
improve outcome (Clay, 2000). This type of monitoring appears beneficial in the
management of patients at risk for cere-bral ischemia; however, the invasive
nature of this type of moni-toring and current limitations in technology
mandate caution in its use. More study is needed before SjO2
monitoring can be con-sidered a valid and reliable tool for the management of
cerebral ischemia (Clay, 2000).
Related Topics