Chapter: Basic & Clinical Pharmacology : Hypothalamic & Pituitary Hormones
Growth Hormone (Somatotropin)
GROWTH HORMONE (SOMATOTROPIN)
Growth hormone, an
anterior pituitary hormone, is required during childhood and adolescence for
attainment of normal adult size and has important effects throughout postnatal
life on lipid and carbohydrate metabolism, and on lean body mass and bone
den-sity. Its growth-promoting effects are primarily mediated via IGF-I(also known as somatomedin C). Individuals with congenital oracquired deficiency
of GH during childhood or adolescence fail to reach their predicted adult
height and have disproportionately increased body fat and decreased muscle
mass. Adults with GH deficiency also have disproportionately low lean body
mass.
Chemistry & Pharmacokinetics
A. Structure
Growth hormone is a
191-amino-acid peptide with two sulfhydryl bridges. Its structure closely
resembles that of prolactin. In the past, medicinal GH was isolated from the
pituitaries of human cadavers. However, this form of GH was found to be
contami-nated with prions that could cause Creutzfeldt-Jakob disease. For this
reason, it is no longer used. Somatropin,
the recombinant form of GH, has a 191-amino-acid sequence that is identical
with the predominant native form of human GH.
B. Absorption, Metabolism, and Excretion
Circulating endogenous
GH has a half-life of 20–25 minutes and is predominantly cleared by the liver.
Recombinant human GH (rhGH) is administered subcutaneously 6–7 times per week.
Peak levels occur in 2–4 hours and active blood levels persist for
approximately 36 hours.
Pharmacodynamics
Growth
hormone mediates its effects via cell surface receptors of the JAK/STAT
cytokine receptor superfamily. Growth hormone has two distinct GH receptor
binding sites. Dimerization of two GH receptors is stimulated by a single GH
molecule and acti-vates signaling cascades mediated by receptor-associated JAK
tyrosine kinases and STATs . GH has complex effects on growth, body
composition, and carbohydrate, protein, and lipid metabolism. The
growth-promoting effects are medi-ated principally through an increase in the
production of IGF-I. Much of the circulating IGF-I is produced in the liver. GH
also stimulates production of IGF-I in bone, cartilage, muscle, kid-ney, and
other tissues, where it plays autocrine or paracrine roles. GH stimulates
longitudinal bone growth until the epiphyses close—near the end of puberty. In
both children and adults, GH has anabolic effects in muscle and catabolic
effects in lipid cells that shift the balance of body mass to an increase in
muscle mass and a reduction in adiposity. The direct and indirect effects of GH
on carbohydrate metabolism are mixed, in part because GH and IGF-I have
opposite effects on insulin sensitivity. GH reduces insulin sensitivity, which
results in mild hyperinsulin-emia, whereas IGF-I has insulin-like effects on
glucose transport. In patients who are unable to respond to GH because of
severe GH resistance (caused by GH receptor mutations, post-GH receptor
signaling mutations, or GH antibodies), the administra-tion of recombinant
human IGF-I may cause hypoglycemia because of its insulin-like effects.
Clinical Pharmacology
A. Growth Hormone Deficiency
GH deficiency can have
a genetic basis, may be associated with midline developmental defect syndromes
(eg, septo-optic dysplasia), or can be acquired as a result of damage to the
pituitary or hypothalamus by a trauma (including breech or traumatic delivery),
intracranial tumors, infection, infiltrative or hemorrhagic pro-cesses, or
irradiation. Neonates with isolated GH deficiency are typically of normal size
at birth because prenatal growth is not GH-dependent. In contrast, IGF-I is
essential for normal prenatal and postnatal growth. Through poorly understood
mechanisms, IGF-I expression and postnatal growth become GH-dependent during
the first year of life. In childhood, GH deficiency typically presents as short
stature, often with mild adiposity. Another early sign of GH deficiency is
hypoglycemia due to unopposed action of insulin, to which young children are especially
sensitive. Criteria for diagnosis of GH deficiency usually include (1) a
subnormal height velocity for age and (2) a subnormal serum GH response
following stimulation with at least two GH secretagogues. The prevalence of GH
deficiency is approximately 1:5000. Therapy with rhGH permits many children
with short stature due to GH deficiency to achieve normal adult height.
In the past, it was
believed that adults with GH deficiency do not exhibit a significant syndrome.
However, more detailed studies suggest that adults with GH deficiency often
have gener-alized obesity, reduced muscle mass, asthenia, and reduced car-diac
output. GH-deficient adults who have been treated with GH have been shown to
experience a reversal of many of these manifestations.
B. Growth Hormone Treatment of Pediatric Patients with Short Stature
Although the greatest
improvement in growth occurs in patients with GH deficiency, exogenous GH has
some effect on height in
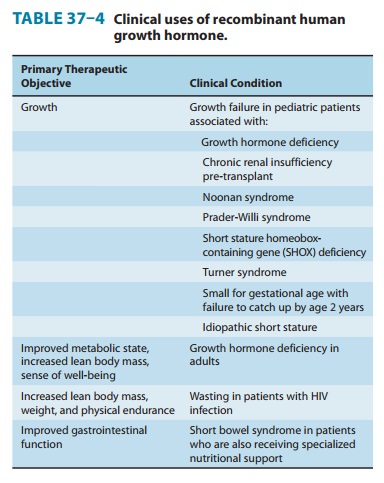
GH has been approved for several
conditions (Table 37–4) and has been used experimentally or off-label in many
others. Prader-Willi syndrome is an
autosomal dominant genetic diseaseassociated with growth failure, obesity, and
carbohydrate intoler-ance. In children with Prader-Willi syndrome and growth
failure, GH treatment decreases body fat and increases lean body mass, linear
growth, and energy expenditure.
GH
treatment has also been shown to have a strong beneficial effect on final
height of girls with Turner syndrome
(45 X karyo-type and variants). In clinical trials, GH treatment has been shown
to increase final height in girls with Turner syndrome by 10–15 cm (4–6
inches). Because girls with Turner syndrome also have either absent or
rudimentary ovaries, GH must be judiciously combined with gonadal steroids to
achieve maximal height. Other condi-tions of pediatric growth failure for which
GH treatment is FDA-approved include chronic renal insufficiency pre-transplant
and small-for-gestational-age at birth in which the child’s height remains more
than 2 standard deviations below the norm at 2 years of age.
A
controversial but approved use of GH is for children with idiopathic short
stature (ISS). This is a heterogeneous popula-tion that has in common no
identifiable cause of the short stature. Some have arbitrarily defined ISS
clinically as having a height at least 2.25 standard deviations below the norm
for children of the same age and an unlikelihood of achieving an adult height
that will be less than 2.25 standard deviations below the norm. In this group
of children, many years of GH therapy result in an average increase in adult
height of 4–7 cm (1.57–2.76 inches) at a cost of $5000–$40,000 per year. The
complex issues involved in the cost-risk-benefit relationship of this use of GH
are important because an estimated 400,000 children in the United States fit
the diag-nostic criteria for ISS.
Treatment
of children with short stature should be carried out by specialists experienced
in GH administration. GH dose require-ments vary with the condition being
treated, with GH-deficient children typically being most responsive. Children
must be observed closely for slowing of growth velocity, which could indicate a
need to increase the dosage or the possibility of epiphyseal fusion or
intercurrent problems such as hypothyroidism or malnutrition.
Other Uses of Growth Hormone
Growth hormone affects
many organ systems and also has a net anabolic effect. It has been tested in a
number of conditions that are associated with a severe catabolic state and is
approved for the treatment of wasting in patients with AIDS. In 2004, GH was
approved for treatment of patients with short bowel syndrome who are dependent
on total parenteral nutrition (TPN). After intestinal resection or bypass, the
remaining functional intestine in many patients undergoes extensive adaptation
that allows it to adequately absorb nutrients. However, other patients fail to
ade-quately adapt and develop a malabsorption syndrome. GH has been shown to
increase intestinal growth and improve its function in experimental animals.
Benefits of GH treatment for patients with short bowel syndrome and dependence
on total parenteral nutrition have mostly been short-lived in the clinical
studies that have been published to date. Growth hormone is administered with
glutamine, which also has trophic effects on the intestinal mucosa.
GH is a popular
component of “anti-aging” programs. Serum levels of GH normally decline with
aging; anti-aging programs claim that injection of GH or administration of
drugs purported to increase GH release are effective anti-aging remedies. These
claims are largely unsubstantiated. In contrast studies in mice and the
nematode C elegans have clearly
demonstrated that ana-logs of human GH and IGF-I consistently shorten life span and that
loss-of-function mutations in the signaling pathways for the GH and IGF-I
analogs lengthen life span. Another use of GH is by athletes for a purported
increase in muscle mass and athletic performance. GH is one of the drugs banned
by the Olympic Committee.
Although GH has
important effects on lipid and carbohydrate metabolism and on lean body mass,
it does not seem likely to be a fruitful direct target for efforts to develop
new drugs to treat obe-sity. However, some of the hormonal and neuroendocrine
systems that regulate GH secretion are being investigated as possible targets
for antiobesity drugs (see Box: Treatment of Obesity).
In 1993, the FDA approved the use of recombinant bovine growth hormone (rbGH) in dairy cattle to increase milk produc-tion. Although milk and meat from rbGH-treated cows appear to be safe, these cows have a higher incidence of mastitis, which could increase antibiotic use and result in greater antibiotic resi-dues in milk and meat.
Toxicity & Contraindications
Children generally tolerate GH treatment well. Adverse events are relatively rare and include pseudotumor cerebri; slipped capital femoral epiphysis; progression of scoliosis; edema; hyperglycemia; and increased risk of asphyxiation in severely obese patients with Prader-Willi syndrome and upper airway obstruction or sleep apnea. Patients with Turner syndrome have an increased risk of otitis media while taking GH. In children with GH deficiency, periodic evaluation of the other anterior pituitary hormones may reveal concurrent defi-ciencies, which also require treatment (eg, with hydrocortisone, levothyroxine, or gonadal hormones). Pancreatitis, gynecomastia, and nevus growth have occurred in patients receiving GH. Adults tend to have more adverse effects from GH therapy. Peripheral edema, myalgias, and arthralgias (especially in the hands and wrists) occur commonly but remit with dosage reduction. Carpal tunnel syndrome can occur. GH treatment increases the activity of cyto-chrome P450 isoforms, which may reduce the serum levels of drugs metabolized by that enzyme system .
There has been no increased incidence of malignancy among
patients receiving GH therapy, but GH treatment is contraindicated in a patient
with a known active malignancy. Proliferative retinopathy may rarely occur. GH
treatment of critically ill patients appears to increase mortality.
Related Topics