Chapter: Basic & Clinical Pharmacology : Hypothalamic & Pituitary Hormones
Gonadotropin-Releasing Hormone & Its Analogs
GONADOTROPIN-RELEASING HORMONE
& ITS ANALOGS
Gonadotropin-releasing
hormone is secreted by neurons in the hypothalamus. It travels through the
hypothalamic-pituitary venous portal plexus to the anterior pituitary, where it
binds to G protein-coupled receptors on the plasma membranes of gonado-troph
cells. Pulsatile GnRH secretion is
required to stimulate the gonadotroph cell to produce and release LH and FSH.Sustained
nonpulsatile administration of GnRH
or GnRH analogs inhibits the release
of FSH and LH by the pituitary in both women and men, resulting in
hypogonadism. GnRH ago-nists are used to produce gonadal suppression in men
with pros-tate cancer. They are also used in women who are undergoing assisted
reproductive technology procedures or who have a gyne-cologic problem that is
benefited by ovarian suppression.
Chemistry & Pharmacokinetics
A. Structure
GnRH is a decapeptide
found in all mammals. Gonadorelin is
an acetate salt of synthetic human GnRH. Synthetic analogs of GnRH include goserelin, histrelin, leuprolide,
nafarelin, and triptorelin. These
analogs all have D-amino acids at position 6,and all but nafarelin have
ethylamide substituted for glycine at position 10. Both modifications make them
more potent and longer-lasting than native GnRH and gonadorelin.
B. Pharmacokinetics
Gonadorelin can be
administered intravenously or subcutane-ously. GnRH analogs can be administered
subcutaneously, intra-muscularly, via nasal spray (nafarelin), or as a
subcutaneous implant. The half-life of intravenous gonadorelin is 4 minutes,
and the half-lives of subcutaneous and intranasal GnRH analogs are
approximately 3 hours. The duration of clinical uses of GnRH agonists varies
from a few days for ovulation induction to a num-ber of years for treatment of
metastatic prostate cancer. Therefore,preparations have been developed with a
range of durations of action from several hours (for daily administration) to
1, 4, 6, or 12 months (depot forms).
Pharmacodynamics
The physiologic
actions of GnRH exhibit complex dose-response relationships that change
dramatically from the fetal period through the end of puberty. This is not
surprising in view of the complex role that GnRH plays in normal reproduction,
particu-larly in female reproduction. Pulsatile GnRH release occurs and is
responsible for stimulating LH and FSH production during the fetal and neonatal
period. Subsequently, from the age of 2 years until the onset of puberty, GnRH
secretion falls off and the pitu-itary simultaneously exhibits very low
sensitivity to GnRH. Just before puberty, an increase in the frequency and
amplitude of GnRH release occurs and then, in early puberty, pituitary sensitiv-ity
to GnRH increases, which is due in part to the effect of increas-ing
concentrations of gonadal steroids. In females, it usually takes several months
to a year after the onset of puberty for the hypo-thalamic-pituitary system to
produce an LH surge and ovulation. By the end of puberty, the system is well
established so that men-strual cycles proceed at relatively constant intervals.
The ampli-tude and frequency of GnRH pulses vary in a regular pattern through
the menstrual cycle with the highest amplitudes occur-ring during the luteal
phase and the highest frequency occurring late in the follicular phase. Lower
pulse frequencies favor FSH secretion, whereas higher pulse frequencies favor
LH secretion. Gonadal steroids as well as the peptide hormones activin and
inhibin have complex modulatory effects on the gonadotropin response to GnRH.
In the pharmacologic
use of GnRH and its analogs, pulsatile intravenous administration of
gonadorelin every 1–4 hours stimu-lates FSH and LH secretion. Continuous
administration of gona-dorelin or its longer-acting analogs produces a biphasic
response. During the first 7–10 days, an agonist effect results in increased
concentrations of gonadal hormones in males and females; this initial phase is
referred to as a flare. After this
period, the contin-ued presence of GnRH results in an inhibitory action that
mani-fests as a drop in the concentration of gonadotropins and gonadal
steroids. The inhibitory action is due to a combination of receptor
down-regulation and changes in the signaling pathways activated by GnRH.
Clinical Pharmacology
The GnRH agonists are
occasionally used for stimulation of gonadotropin production. They are used far
more commonly for suppression of gonadotropin release.
A. Stimulation
Female
infertility—In
the current era of widespread avail-ability of gonadotropins and assisted
reproductive technology, the use of pulsatile GnRH administration to treat
infertility is uncom-mon. Although pulsatile GnRH is less likely than
gonadotropins
to cause multiple pregnancies
and the ovarian hyperstimulation syndrome, the inconvenience and cost
associated with continuous use of an intravenous pump and difficulties
obtaining native GnRH (gonadorelin) are barriers to pulsatile GnRH. When this
approach is used, a portable battery-powered programmable pump and intravenous
tubing deliver pulses of gonadorelin every 90 minutes.
Gonadorelin or a GnRH
agonist analog can be used to initiate an LH surge and ovulation in women with
infertility who are undergoing ovulation induction with gonadotropins.
Traditionally, hCG has been used to initiate ovulation in this situation.
However, there is some evidence that gonadorelin or a GnRH agonist is less
likely than hCG to cause multiple ova to be released and less likely to cause
the ovarian hyperstimulation syndrome.
2. Male infertility—It is possible to use
pulsatile gonadorelinfor infertility in men with hypothalamic hypogonadotropic
hypogonadism. A portable pump infuses gonadorelin intrave-nously every 90
minutes. Serum testosterone levels and semen analyses must be done regularly.
At least 3–6 months of pulsatile infusions are required before significant
numbers of sperm are seen. As described above, treatment of hypogonadotropic
hypogo-nadism is more commonly done with hCG and hMG or their recombinant
equivalents.
3. Diagnosis of LH
responsiveness— GnRH can be useful indetermining
whether delayed puberty in a hypogonadotropic adolescent is due to
constitutional delay or to hypogonadotropic hypogonadism. The LH response (but
not the FSH response) to a single dose of GnRH can distinguish between these
two condi-tions. Serum LH levels are measured before and at various times after
an intravenous or subcutaneous bolus of GnRH. An increase in serum LH with a
peak that exceeds 15.6 mIU/mL is normal and suggests impending puberty. An
impaired LH response sug-gests hypogonadotropic hypogonadism due to either
pituitary or hypothalamic disease, but does not rule out constitutional delay
of adolescence.
B. Suppression of Gonadotropin Production
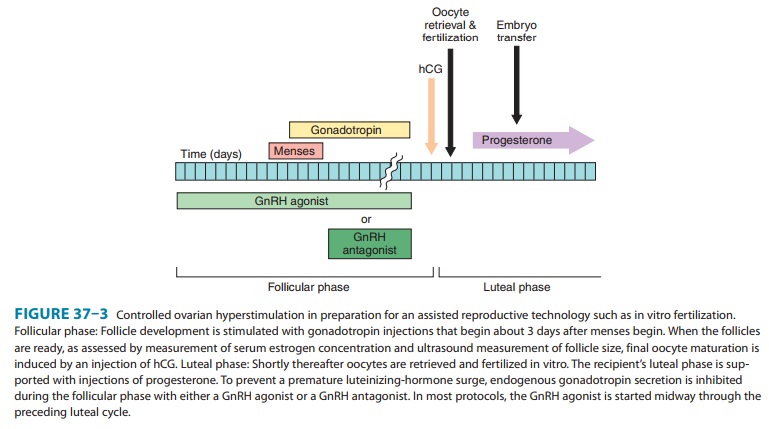
1. Controlled ovarian
hyperstimulation—In the con-trolled ovarian
hyperstimulation that provides multiple mature oocytes for assisted
reproductive technologies such as in vitro fertilization, it is critical to
suppress an endogenous LH surge that could prematurely trigger ovulation. This
suppression is most commonly achieved by daily subcutaneous injections of
leuprolide or daily nasal applications of nafarelin. For leuprolide, treatment
is commonly initiated with 1.0 mg daily for about 10 days or until menstrual
bleeding occurs. At that point, the dose is reduced to 0.5 mg daily until hCG
is administered (Figure 37–3). For nafarelin, the beginning dosage is generally
400 mcg twice a day, which is decreased to 200 mcg when menstrual bleeding
occurs. In women who respond poorly to the standard protocol, alternative
protocols that use shorter courses and lower doses of GnRH agonists may improve
the follicular response to gonadotropins.
2. Endometriosis—Endometriosis is a
syndrome of cyclicalabdominal pain in premenopausal women that is due to the
pres-ence of estrogen-sensitive endometrium-like tissue outside the uterus. The
pain of endometriosis is often reduced by abolishing exposure to the cyclical
changes in the concentrations of estrogen and progesterone that are a normal
part of the menstrual cycle. The ovarian suppression induced by continuous
treatment with a GnRH agonist greatly reduces estrogen and progesterone
concen-trations and prevents cyclical changes. The preferred duration of
treatment with a GnRH agonist is limited to 6 months because ovarian
suppression beyond this period can result in decreased bone density.
Leuprolide, goserelin, and nafarelin are approved for this indication.
Leuprolide and goserelin are administered as depot preparations that provide 1
or 3 months of continuous GnRH agonist activity. Nafarelin is administered
twice daily as a nasal spray at a dose of 0.2 mg per spray.
3. Uterine leiomyomata (uterine
fibroids)—Uterine
leio-myomata are benign, estrogen-sensitive, fibrous growths in the uterus that
can cause menorrhagia, with associated anemia and pelvic pain. Treatment for
3–6 months with a GnRH agonist reduces fibroid size and, when combined with
supplemental iron, improves anemia. Leuprolide, goserelin, and nafarelin are
approved for this indication. The doses and routes of administration are
similar to those described for treatment of endometriosis.
4. Prostate cancer—Antiandrogen therapy is
the primarymedical therapy for prostate cancer. Combined antiandrogen therapy
with continuous GnRH agonist and an androgen receptor antagonist such as
flutamide is as effective as surgical
castration in reducing serum testosterone concentrations and effects.
Leuprolide, goserelin, histrelin, and triptorelin are approved for this
indication. The preferred formulation is one of the long-acting depot forms
that provide 1, 3, 4, 6, or 12 months of active drug therapy. During the first
7–10 days of GnRH ana-log therapy, serum testosterone levels increase because
of the ago-nist action of the drug; this can precipitate pain
in patients with bone metastases, and tumor growth and neurologic symptoms in
patients with vertebral metastases. It can also temporarily worsen symptoms of
urinary obstruction. Such tumor flares can usually be avoided with the
concomitant administration of bicalutamide or one of the other androgen
receptor antagonists . Within about 2 weeks, serum testosterone levels fall to
the hypo-gonadal range.
5. Central
precocious puberty— Continuous administrationof a GnRH agonist is indicated for
treatment of central precocious puberty (onset of secondary sex characteristics
before 7–8 years in girls or 9 years in boys). Before embarking on treatment
with a GnRH agonist, one must confirm central precocious puberty by
demonstrating a pubertal gonadotropin response to GnRH or a “test dose” of a
GnRH analog. Treatment is typically indicated in a child whose final height
would be otherwise significantly com-promised (as evidenced by a significantly
advanced bone age) or in whom the development of pubertal secondary sexual
characteristicsor menses causes significant emotional distress. While central
precocious puberty is most often idiopathic, it is important to rule out
central nervous system pathology with MRI imaging of the hypothalamic-pituitary
area.
Treatment is most
commonly carried out with either a monthly intramuscular depot injection of
leuprolide acetate or with a once-yearly implant of histrelin acetate. Daily
subcutaneous regimens and multiple daily nasal spray regimens of GnRH agonists
are also available. Treatment with a GnRH agonist is generally continued to age
11 in females and age 12 in males.
6. Other—Other clinical uses
for the gonadal suppression pro-vided by continuous GnRH agonist treatment
include advanced breast and ovarian cancer; thinning of the endometrial lining
in preparation for an endometrial ablation procedure in women with
dysfunctional uterine bleeding; and treatment of amenorrhea and infertility in
women with polycystic ovary disease. Recently pub-lished clinical practice
guidelines recommend the use of continu-ous GnRH agonist administration in
early pubertal transgender adolescents to block endogenous puberty prior to
subsequent treatment with cross-gender gonadal hormones.
Toxicity
Gonadorelin can cause
headache, light-headedness, nausea, and flushing. Local swelling often occurs
at subcutaneous injection sites. Generalized hypersensitivity dermatitis has
occurred after long-term subcutaneous administration. Rare acute
hypersensitiv-ity reactions include bronchospasm and anaphylaxis. Sudden
pituitary apoplexy and blindness have been reported following administration of
GnRH to a patient with a gonadotropin-secret-ing pituitary tumor.
Continuous treatment
of women with a GnRH analog (leuprolide, nafarelin, goserelin) causes the
typical symptoms of menopause, which include hot flushes, sweats, and
headaches. Depression, diminished libido, generalized pain, vaginal dryness,
and breast atrophy may also occur. Ovarian cysts may develop within the first 2
months of therapy and generally resolve after an additional 6 weeks; however,
the cysts may persist and require discontinuation of therapy. Reduced bone
density and osteoporo-sis may occur with prolonged use, so patients should be
monitored with bone densitometry before repeated treatment courses. Depending
on the condition being treated with the GnRH ago-nist, it may be possible to
ameliorate the signs and symptoms of the hypoestrogenic state without losing
clinical efficacy by adding back a small dose of a progestin alone or in
combination with a low dose of an estrogen. Contraindications to the use of
GnRH agonists in women include pregnancy and breast-feeding.
In men treated with
continuous GnRH agonist administra-tion, adverse effects include hot flushes
and sweats, edema, gyne-comastia, decreased libido, decreased hematocrit,
reduced bone density, asthenia, and injection site reactions. GnRH analog
treatment of children is generally well tolerated. However, tem-porary
exacerbation of precocious puberty may occur during the first few weeks of
therapy. Nafarelin nasal spray may cause or aggravate sinusitis.
Related Topics