Chapter: Basic & Clinical Pharmacology : Hypothalamic & Pituitary Hormones
GNRH Receptor Antagonists
GNRH RECEPTOR ANTAGONISTS
Three synthetic
decapeptides that function as competitive antagonists of GnRH receptors are
available for clinical use. Ganirelix, cetrorelix,
and degarelix inhibit the
secretion of FSH and LH in dose-dependent manner. Ganirelix and cetrorelix are
approved for use in controlled ovarian hyperstimulation procedures, whereas
degarelix is approved for men with advanced prostate cancer.
Pharmacokinetics
Ganirelix and
cetrorelix are absorbed rapidly after subcutaneous injection. Administration of
0.25 mg daily maintains GnRH antagonism. Alternatively, a single 3.0-mg dose of
cetrorelix sup-presses LH secretion for 96 hours. Degarelix therapy is
initiated with 240 mg administered as two subcutaneous injections. Maintenance
dosing is with an 80-mg subcutaneous injection every 28 days.
Clinical Pharmacology
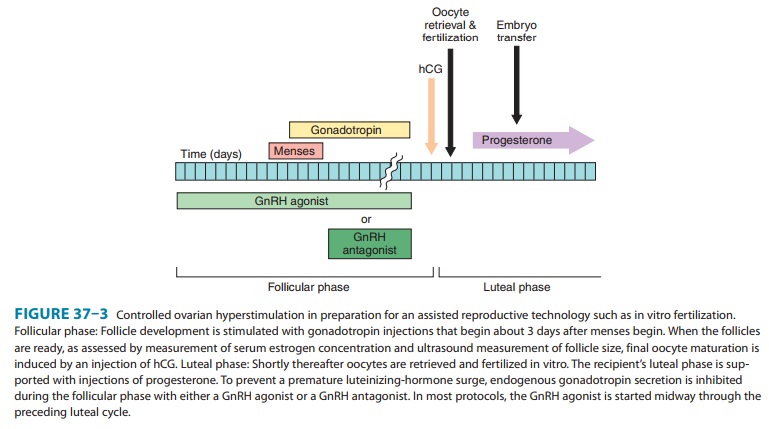
A. Suppression of Gonadotropin Production
GnRH antagonists are
approved for preventing the LH surge dur-ing controlled ovarian
hyperstimulation. They offer several advan-tages over continuous treatment with
a GnRH agonist. Because GnRH antagonists produce an immediate antagonist
effect, their use can be delayed until day 6–8 of the in vitro fertilization
cycle (Figure 37–3), and thus the duration of administration is shorter. They
also appear to have a less negative impact on the ovarian response to
gonadotropin stimulation, which permits a decrease in the total duration and
dose of gonadotropin. Finally, GnRH antagonists are associated with a lower
risk of ovarian hyperstimu-lation syndrome, which can lead to cycle
cancellation. On the other hand, because their antagonist effects reverse more
quickly after their discontinuation, adherence to the treatment regimen is
critical. The antagonists produce a more complete suppression of gonadotropin
secretion than agonists. There is concern that the suppression of LH may
inhibit ovarian steroidogenesis to an extent that impairs follicular
development when recombinant or the puri-fied form of FSH is used during the
follicular phase of an in vitro fertilization cycle. Clinical trials have shown
a slightly lower rate of pregnancy in in vitro fertilization cycles that used
GnRH antago-nist treatment compared with cycles that used GnRH agonist
treatment.
B. Advanced Prostate Cancer
Degarelix is approved
for the treatment of symptomatic advanced prostate cancer. This GnRH antagonist
reduces concentrations of gonadotropins and androgens more rapidly than GnRH
ago-nists and avoids the testosterone surge seen with GnRH agonist therapy.
Toxicity
When used for
controlled ovarian hyperstimulation, ganirelix and cetrorelix are well
tolerated. The most common adverse effects are nausea and headache. During the
treatment of men with prostate cancer, degarelix caused injection-site
reactions and increases in liver enzymes. Like continuous treatment with a GnRH
agonist, degarelix leads to signs and symptoms of androgen deprivation,
including hot flushes and weight gain.
Related Topics