Chapter: Basic & Clinical Pharmacology : Hypothalamic & Pituitary Hormones
Dopamine Agonists
DOPAMINE AGONISTS
Adenomas that secrete
excess prolactin usually retain the sensitiv-ity to inhibition by dopamine
exhibited by the normal pituitary. Bromocriptine
and cabergoline are ergot
derivatives with a high affinity for
dopamine D2 recep-tors. Quinagolide, a drug approved in Europe,
is a nonergot agent with similarly high D 2 receptor affinity.
Dopamine agonists
suppress prolactin release very effectively in patients with
hyperprolactinemia. GH release is reduced in patients with acromegaly, although
not as effectively. Bromocriptine has also been used in Parkinson’s disease to
improve motor function and reduce levodopa requirements . Newer, nonergot D2 agonists used in
Parkinson’s disease (pramipexole and ropinirole;) have been reported to
interfere with lactation, but they are not approved for use in
hyperprolactinemia.
Pharmacokinetics
All
available dopamine agonists are active as oral preparations, and all are
eliminated by metabolism. They can also be absorbed systemically after vaginal
insertion of tablets. Cabergoline, with a half-life of approximately 65 hours,
has the longest duration of action. Quinagolide has a half-life of about 20
hours, whereas the half-life of bromocriptine is about 7 hours. After vaginal
adminis-tration, serum levels peak more slowly.
Clinical Pharmacology
A. Hyperprolactinemia
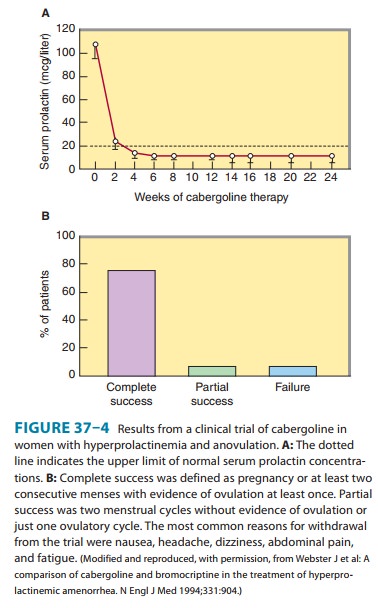
A dopamine agonist is
the standard medical treatment for hyper-prolactinemia. These drugs shrink
pituitary prolactin-secreting tumors, lower circulating prolactin levels, and
restore ovulation in approximately 70% of women with microadenomas and 30% of
women with macroadenomas (Figure 37–4). Cabergoline is initi-ated at 0.25 mg
twice weekly orally or vaginally. It can be increased gradually, according to
serum prolactin determinations, up to a maximum of 1 mg twice weekly.
Bromocriptine is generally taken daily after the evening meal at the initial
dose of 1.25 mg; the dose
is then increased as
tolerated. Most patients require 2.5–7.5 mg daily. Long-acting oral
bromocriptine formulations (Parlodel SRO) and intramuscular formulations
(Parlodel L.A.R.) are available outside the United States.
B. Physiologic Lactation
Dopamine agonists were
used in the past to prevent breast engorgement when breast-feeding was not
desired. Their use for this purpose has been discouraged because of toxicity
(see Toxicity & Contraindications).
C. Acromegaly
A dopamine agonist
alone or in combination with pituitary sur-gery, radiation therapy, or
octreotide administration can be used to treat acromegaly. The doses required
are higher than those used to treat hyperprolactinemia. For example, patients with
acromeg-aly require 20–30 mg/d of bromocriptine and seldom respond adequately
to bromocriptine alone unless the pituitary tumor secretes prolactin as well as
GH.
Toxicity & Contraindications
Dopamine agonists can
cause nausea, headache, light-headedness, orthostatic hypotension, and fatigue.
Psychiatric manifestations occasionally occur, even at lower doses, and may
take months to resolve. Erythromelalgia occurs rarely. High dosages of
ergot-derived preparations can cause cold-induced peripheral digital vasospasm.
Pulmonary infiltrates have occurred with chronic high-dosage therapy.
Cabergoline appears to cause nausea less often than bromocriptine. Vaginal
administration can reduce nausea, but may cause local irritation.
Dopamine agonist
therapy during the early weeks of pregnancy has not been associated with an
increased risk of spontaneous abortion or congenital malformations. Although
there has been a longer experience with the safety of bromocriptine during
early pregnancy, there is growing evidence that cabergoline is also safe in
women with macroadenomas who must continue a dopamine agonist during pregnancy.
In patients with small pituitary ade-nomas, dopamine agonist therapy is
discontinued upon concep-tion because growth of microadenomas during pregnancy
is rare. Patients with very large adenomas require vigilance for tumor
progression and often require a dopamine agonist throughout pregnancy. There
have been rare reports of stroke or coronary thrombosis in postpartum women
taking bromocriptine to sup-press postpartum lactation.
Related Topics