Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Management of Patients with Fluid & Electrolyte Disturbances
Fluid Compartments
Fluid Compartments
Body water is distributed between two major fluid compartments separated
by cell membranes: intra-cellular fluid (ICF) and extracellular fluid (ECF).
The latter can be further subdivided into intra-vascular and interstitial
compartments. The inter-stitium includes all fluid that is both outside cells
and outside the vascular endothelium. The relative contributions of each
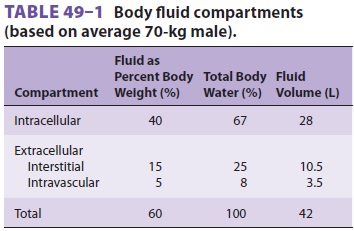
compartment to total body water (TBW) and body weight are delineated in Table
49–1.
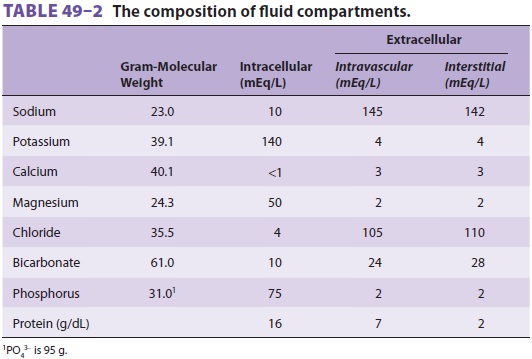
The volume of fluid (water) within a
compart-ment is determined by its solute composition and concentrations (Table
49–2). Differences in solute concentrations are
largely due to the characteristics of the physical barriers that separate
compartments . The osmotic forces created by “trapped” solutes govern the
distribution of water between com-partments and ultimately each compartment’s
volume.
INTRACELLULAR FLUID
The outer
membrane of cells plays an important role in regulating intracellular volume
and compo-sition. A membrane-bound adenosine triphosphate (ATP)–dependent pump
exchanges Na+ for K+ in a 3:2 ratio. Because cell membranes are relatively impermeable to
sodium and (to a lesser extent) potassium ions, potassium is concentrated
intracellularly, whereas sodium is concentrated extra-cellularly. As a result,
potassium is the mostimportant determinant of intracellular osmotic pressure,
whereas sodium is the most important determinant of extracellular osmotic
pressure.
The impermeability of cell membranes to most
proteins results in a high intracellular protein con-centration. Because
proteins act as nondiffusible sol-utes (anions), the unequal exchange ratio of
3 Na+ for 2 K+ by the cell membrane
pump is critical in preventing relative intracellular hyperosmolality.
Interference with Na+–K+-ATPase activity, as occurs during ischemia or hypoxia, results in
progressive swelling of cells.
EXTRACELLULAR FLUID
The principal function of ECF is to provide a medium for delivery of cell nutrients and electro-lytes and for removal of cellular waste products. Maintenance of a normal extracellular volume— particularly the circulating component (intravascu-lar volume)—is critical. For the reasons described above, sodium is quantitatively the most important extracellular cation and the major determinant of extracellular osmotic pressure and volume. Changes in ECF volume are therefore related to changes in total body sodium content. The latter is a function of sodium intake, renal sodium excretion, and extrare-nal sodium losses .
Interstitial Fluid
Very little interstitial fluid is normally in
the form of free fluid. Most interstitial water is in chemical asso-ciation
with extracellular proteoglycans, forming a gel. Interstitial fluid pressure is
generally thought to be negative (about −5 mm Hg). As interstitial fluid volume increases, interstitial pressure
also rises and eventually becomes positive. When the latter occurs, the free
fluid in the gel increases rapidly and appears clinically as edema.
Because only small quantities of plasma pro-teins can normally cross
capillary clefts, the pro-tein content of interstitial fluid is relatively low
(2 g/dL). Protein entering the interstitial space is returned to the vascular
system via the lymphatic system.
Intravascular Fluid
Intravascular fluid, commonly referred to as plasma, is restricted to
the intravascular space by the vas-cular endothelium. Most electrolytes (small
ions) freely pass between plasma and the interstitium, resulting in nearly
identical electrolyte composi-tion. However, the tight intercellular junctions
between adjacent endothelial cells impede the pas-sage of plasma proteins to
outside the intravascular compartment. As a result, plasma proteins (mainly
albumin) are the only osmotically active solutes in fluid not normally
exchanged between plasma and interstitial fluid.
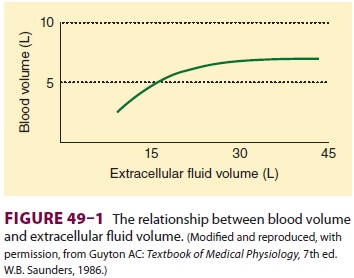
Increases in extracellular volume are normally proportionately reflected
in intravascular and inter-stitial volume. However, when interstitial pressure
becomes positive, continued increases in ECF result in expansion of only the
interstitial fluid compart-ment (Figure 49–1). In this way, the
interstitial compartment acts as an overflow reservoir for the intravascular
compartment. This is seen clinically in the form of tissue edema.
EXCHANGE BETWEEN FLUID COMPARTMENTS
Diffusion is the random movement of molecules
due to their kinetic energy and is responsible for the majority of fluid and
solute exchange between com-partments. The rate of diffusion of a substance
across a membrane depends upon (1) the permeability of that substance through
that membrane, (2) the con-centration difference for that substance between the
two sides, (3) the pressure difference between either side because pressure
imparts greater kinetic energy, and (4) the electrical potential across the
membrane for charged substances.
Diffusion Through Cell Membranes
Diffusion between interstitial fluid and ICF may
take place by one of several mechanisms: (1) directly through the lipid bilayer
of the cell mem-brane, (2) through protein channels within the membrane, or (3)
by reversible binding to a carrier protein that can traverse the membrane
(facilitated diffusion). Oxygen, CO2, water, and lipid-soluble
molecules penetrate the cell membrane directly. Cations such as Na+, K+, and Ca 2+ penetrate the membrane poorly because of the cell transmem-brane
voltage potential (which is positive to the outside) created by the Na+–K+ pump. Therefore, these cations can diffuse
only through specific pro-tein channels. Passage through these channels is
dependent on membrane voltage and the binding of
ligands (such as acetylcholine) to the membrane receptors. Glucose and amino
acids diffuse with the help of membrane-bound carrier proteins. Fluid exchange
between the intracellular and interstitial spaces is governed by the
osmoticforces created by differences in nondiffusible sol-ute concentrations.
Relative changes in osmolality between the intracellular and interstitial
compart-ments result in a net water movement from the hypoosmolar to the
hyperosmolar compartment.
Diffusion Through Capillary Endothelium
Capillary walls are typically 0.5 µm thick, consist-ing of a single layer of endothelial cells with their
basement membrane. Intercellular clefts, 6–7 nm wide, separate each cell from
its neighbors. Oxygen, CO2, water, and lipid-soluble
substances can pen-etrate directly through both sides of the endothelial cell
membrane. Only low-molecular-weight water-soluble substances such as sodium,
chloride, potas-sium, and glucose readily cross intercellular clefts.
High-molecular-weight substances such as plasma proteins penetrate the
endothelial clefts poorly (except in the liver and the lungs, where the clefts
are larger).
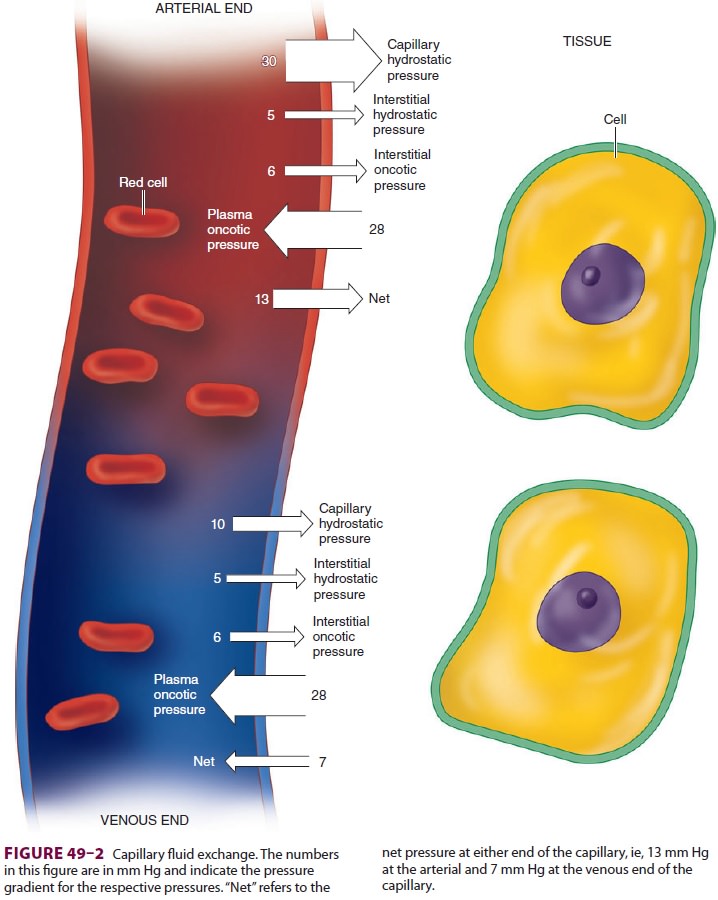
Fluid exchange across capillaries differs from that across cell membranes
in that it is governed by significant differences in hydrostatic pressures in
addition to osmotic forces ( Figure 49–2). These forces are operative on both arterial and venous ends of
capillaries, with a tendency for fluid to move out of capillaries at the
arterial end and back into capillaries at the venous end. Moreover, the
magnitude of these forces differs between the various tissue beds. Arterial
capillary pressure is determined by precapillary sphincter tone. Thus
capillaries that require a high pressure such as glomeruli have low
precapillary sphincter tone, whereas the normally low-pressure capillaries of
muscle have high precapillary sphincter tone. Nor-mally, all but 10% of the
fluid filtered is reabsorbed back into capillaries. What is not reabsorbed
(about 2 mL/min) enters the interstitial fluid and is then returned by
lymphatic flow to the intravas-cular compartment.
Related Topics