Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Management of Patients with Fluid & Electrolyte Disturbances
Disorders of Water Balance
Disorders of Water Balance
The human body at birth is approximately 75%
water by weight. By 1 month this value decreases to 65%, and by adulthood to
60% for males and 50% for females. The higher fat content in females decreases
water content. For the same reason, obesity and advanced age further decrease
water content.
NORMAL WATER BALANCE
The normal adult daily water intake averages 2500 mL, which includes
approximately 300 mL as a byproduct of the metabolism of energy substrates.
Daily water loss averages 2500 mL and is typically accounted for by 1500 mL in
urine, 400 mL in respi-ratory tract evaporation, 400 mL in skin evaporation,
100 mL in sweat, and 100 mL in feces. Evaporative loss is very important in
thermoregulation because this mechanism normally accounts for 20–25% of heat
loss.
Both ICF and ECF osmolalities are tightly regulated to maintain normal
water content in tis-sues. Changes in water content and cell volume can induce
serious impairment of function, particularly in the brain .
RELATIONSHIP OF PLASMA SODIUM CONCENTRATION, EXTRACELLULAR OSMOLALITY, INTRACELLULAR OSMOLALITY
The
osmolality of ECF is equal to the sum of the concentrations of all dissolved
solutes. Because Na + and its anions account for nearly
90% of these sol-utes, the following approximation is valid:
Plasma osmolality = 2 × Plasma sodium
concentration
Moreover,
because ICF and ECF are in osmotic equilibrium, plasma sodium concentration [Na+]plasma generally reflects total body osmolality:
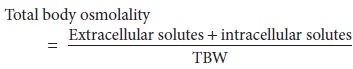
Because
sodium and potassium are the major intra- and extracellular solutes,
respectively:
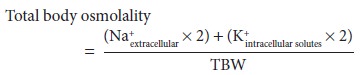
Combining
the two approximations:
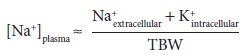
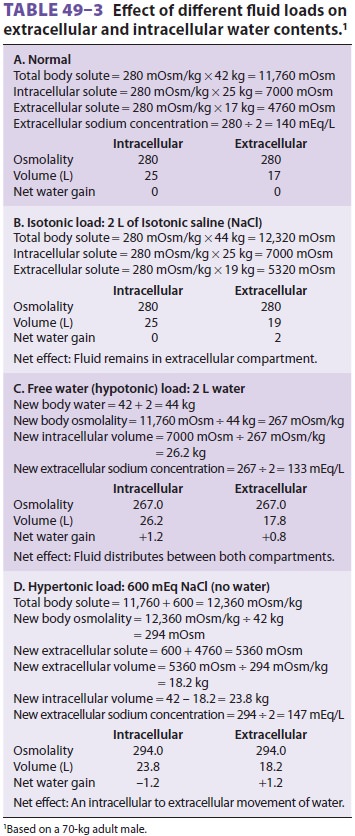
Using these principles, the effect of isotonic,
hypotonic, and hypertonic fluid loads on compart-mental water content and
plasma osmolality can be calculated ( Table 49–3). The potential importance of intracellular
potassium concentration is readily apparent from this equation. Thus
significant potas-sium losses may contribute to hyponatremia.
In
pathological states, glucose and—to a much lesser extent—urea can contribute
significantly to extracellular osmolality. A more accurate approxi-mation of
plasma osmolality is therefore given by the following equation:
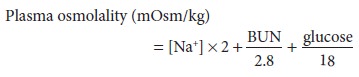
where [Na+] is expressed as mEq/L and blood urea nitrogen (BUN) and glucose as
mg/dL. Urea is an ineffective osmole because it readily permeates cell
membranes and is therefore frequently omitted from this calculation:
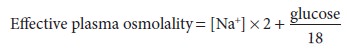
Plasma osmolality normally varies between 280 and 290 mOsm/L. Plasma sodium concentra-tion decreases approximately 1 mEq/L for every 62 mg/dL increase in glucose concentration. A discrepancy between the measured and calculated osmolality is referred to as an osmolal gap. Signifi-cant osmolal gaps indicate a high concentration of an abnormal osmotically active molecule in plasma such as ethanol, mannitol, methanol, ethylene gly-col, or isopropyl alcohol. Osmolal gaps may also be seen in patients with chronic kidney failure (attrib-uted to retention of small solutes), patients with ketoacidosis (as a result of a high concentration of ketone bodies), and those receiving large amounts of glycine (as during transurethral resection of the prostate). Lastly, osmolal gaps may also be present in patients with marked hyperlipidemia or hyper-proteinemia. In such instances, the protein or lipid part of plasma contributes significantly to plasma volume; although plasma [Na+] is decreased, [Na+] in the water phase of plasma (true plasma osmolal-ity) remains normal. The water phase of plasma is normally only 93% of its volume; the remaining 7% consists of plasma lipids and proteins.
Related Topics