Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Management of Patients with Fluid & Electrolyte Disturbances
Disorders of Sodium Balance
Disorders of Sodium Balance
ECF volume is directly proportionate to total
body sodium content. Variations in ECF volume result from changes in total body
sodium content. A posi-tive sodium balance increases ECF volume, whereas a
negative sodium balance decreases ECF volume. It is important to reemphasize
that extracellular(plasma) Na+ concentration is more indicative of water balance than total body
sodium content.
NORMAL SODIUM BALANCE
Net sodium balance is equal to total sodium
intake (adults average 170 mEq/d) minus both renal sodium excretion and
extrarenal sodium losses. (One gram of sodium yields 43 mEq of Na ions,
whereas 1 g of sodium chloride yields 17 mEq of Na+ ions.) The kidneys’ ability to vary urinary
Na + excretion from less than 1 mEq/L to more than 100 mEq/L allows them to
play a critical role in sodium balance .
REGULATION OF SODIUM BALANCE & EXTRACELLULAR FLUID VOLUME
Because of the relationship between ECF volume and total body sodium
content, regulation of one is intimately tied to the other. This regulation is
achieved via sensors that detect changes
in the most important component of ECF, namely, the “effective” intravascular
volume. The latter corre-lates more closely with the rate of perfusion in renal
capillaries than with measurable intravascular fluid (plasma) volume. Indeed,
with edematous disorders (heart failure, cirrhosis, and kidney failure),
“effec-tive” intravascular volume can be independent of the measurable plasma
volume, ECF volume, and even cardiac output.
ECF volume and total body sodium content are
ultimately controlled by appropriate adjustments in renal Na + excretion. In the
absence of kidney disease, diuretic therapy, and selective renal isch-emia,
urinary Na+ concentration reflects “effective” intravascular volume. A low urine Na+ concentra-tion (<10 mEq/L) is therefore
generally indicative of a low “effective” intravascular fluid volume and
reflects secondary retention of Na+ by the kidneys.
Control Mechanisms
The multiple mechanisms involved in regulating ECF volume and sodium
balance normally comple-ment one another but can function independently. In
addition to altering renal Na+ excretion, some mechanisms also
produce more rapid compensatory hemodynamic responses when “effective”
intravas-cular volume is reduced.
A. Sensors of Volume
Baroreceptors are the principal volume
receptors in the body. Because blood pressure is the product of cardiac output
and systemic vascular resistance , significant changes in intravascular vol-ume
(preload) not only affect cardiac output but also transiently affect arterial
blood pressure. Thus, the baroreceptors at the carotid sinus and afferent renal
arterioles (juxtaglomerular apparatus) indirectly function as sensors of
intravascular volume. Changes in blood pressure at the carotid sinus modulate
sym-pathetic nervous system activity and nonosmotic ADH secretion, whereas
changes at the afferent renal arterioles modulate the renin–angiotensin–
aldosterone system. Stretch receptors in both atria are affected by changes in
intravascular volume, and the degree of atrial distention modulates the release
of atrial natriuretic hormone and ADH.
B. Effectors of Volume Change
Regardless of the mechanism, effectors of vol-ume change ultimately
alter urinary Na+
excretion. Decreases in “effective” intravascular volume decrease urinary Na+ excretion, whereas increases
in the “effec-tive” intravascular volume increase urinary Na+ excre-tion. These mechanisms
include the following:
Renin–angiotensin–aldosterone—Renin secre-tion increases the formation of angiotensin II. The latter
increases the secretion of aldosterone and has a direct effect in enhancing Na+ reabsorption in the
proximal renal tubules. Angiotensin II is also a potent direct vasoconstrictor
and potentiates the actions of norepinephrine. Secretion of aldosterone
enhances Na+ reabsorption in the distal nephron
and is a major determinant of uri-nary Na+ excretion.
Atrial
natriuretic peptide (ANP)—This peptideis normally released from both right and left atrial cells
following atrial distention. ANP appears to have two major actions: arterial
vasodilation and increased urinary sodium and water excretion in the renal
collecting tubules. Na +-mediated afferent arte-riolar dilation and efferent arteriolar
constriction can also increase glomerular filtration rate (GFR). Other effects
include the inhibition of both renin and aldosterone secretion and antagonism
of ADH.
Brain natriuretic peptide (BNP)—ANP, BNP, and
C-type natriuretic peptide are structurally related peptides. BNP is released
by the ventricles in response to increased ventricular volume and pressure, and
ventricular overdistention, and also by the brain in response to increased
blood pressure. BNP levels are usually approximately 20% of ANP levels, but
dur-ing an episode of acute congestive heart failure BNP levels may exceed
those of ANP. BNP levels can be measured clinically, and a recombinant form of
BNP, nesiritide (Natrecor), is available to treat acute decompensated
congestive heart failure.
Sympathetic
nervous system
activity—Enhanced
sympathetic activity increases Na+ reab-sorption in the proximal renal
tubules, resulting in Na+
retention, and increases renal vasoconstriction, which reduces renal blood flow
. Conversely, stimulation of left atrial stretch recep-tors results in
decreases in renal sympathetic tone and increases in renal blood flow
(cardiorenal reflex) and glomerular filtration.
Glomerular
filtration rate and plasma sodium concentration—The
amount of Na+fi ltered in thekidneys is directly proportionate to the product of the
GFR and plasma Na+ concentration. Because GFR is usually proportionate to intravascular
vol-ume, intravascular volume expansion can increase Na+ excretion. Conversely,
intravascular volume depletion decreases Na + excretion. Similarly, even small elevations
of blood pressure can result in a relatively large increase in urinary Na+ excretion because of
the resultant increase in renal blood flow and glomerular filtration rate.
Blood pressure– induced diuresis (pressure
natriuresis) appears to be independent of any known humorally or neurally
mediated mechanism.
Tubuloglomerular balance—Despite
wide varia-tions in the amount of Na+ fi ltered in nephrons, Na+ reabsorption in the proximal
renal tubules is normally controlled within narrow limits. Factors considered
to be responsible for tubuloglomerular balance include the rate of renal
tubular flow and changes in peri-tubular capillary hydrostatic and oncotic
pressures. Altered Na+
reabsorption in the proximal tubules can have a marked effect on renal Na+ excretion.
Antidiuretic hormone—Although ADH secre-tion has little effect on Na + excretion, nonosmotic secretion of this hormone (see above) can play an important part in maintaining extracellular volume with moderate to severe decreases in the “effective” intravascular volume.
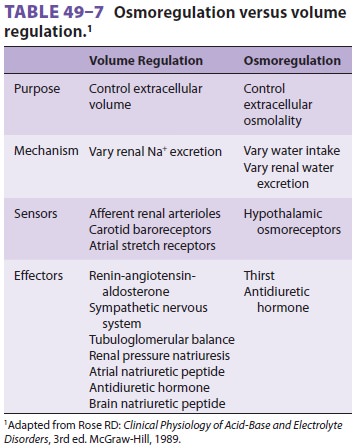
Extracellular Osmoregulation versus Volume Regulation
Osmoregulation protects the normal ratio of
sol-utes to water, whereas extracellular volume regula-tion preserves absolute
solute and water content (Table 49–7).
As noted previously, volume regulation generally takes precedence over
osmoregulation.
Anesthetic Implications
Problems related to altered sodium balance
result from its manifestations as well as the underlying disorder. Disorders of
sodium balance present either as hypovolemia (sodium deficit) or hypervolemia
(sodium excess). Both disturbances should be cor-rected prior to elective
surgical procedures. Cardiac, liver, and renal function should also be
carefully evaluated in the presence of sodium excess (gener-ally manifested as
tissue edema).
Hypovolemic patients are sensitive to the
vaso-dilating and negative inotropic effects of vapor anesthetics, propofol,
and agents associated with histamine release (morphine, meperidine).
Dosagerequirements for other drugs must also be reduced to compensate for
decreases in their volume of dis-tribution. Hypovolemic patients are
particularly sensitive to sympathetic blockade from spinal or epidural
anesthesia. If an anesthetic must be admin-istered prior to adequate correction
of hypovolemia, etomidate or ketamine may be the induction agents of choice for
general anesthesia. Hypervolemia should generally be corrected preoperatively
with diuretics. The major haz-ard of increases in extracellular volume
isimpaired gas exchange due to pulmonary interstitial edema, alveolar edema, or
large collections of pleu-ral or ascitic fluid.
Related Topics