Chapter: Medical Physiology: The Microcirculation and the Lymphatic System: Capillary Fluid Exchange, Interstitial Fluid, and Lymph Flow
Exchange of Water, Nutrients, and Other Substances Between the Blood and Interstitial Fluid
Exchange of Water, Nutrients, and Other Substances Between the Blood and Interstitial Fluid
Diffusion Through the Capillary Membrane
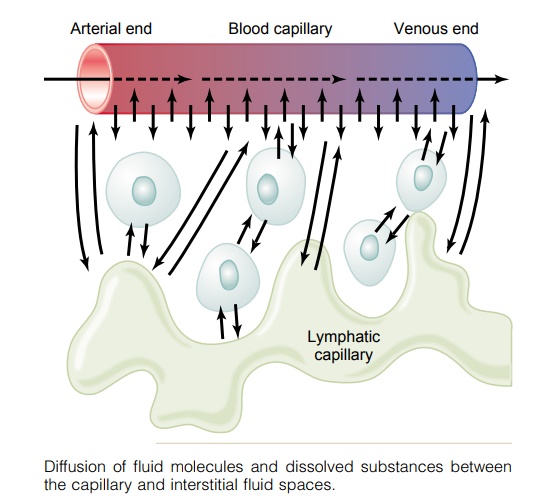
By far the most important means by which substances are transferred between the plasma and the interstitial fluid is diffusion. Figure 16–3 demonstrates this process, showing that as the blood flows along the lumen of the capillary, tremendous numbers of water molecules and dissolved particles diffuse back and forth through the capillary wall, providing continual mixing between the interstitial fluid and the plasma.
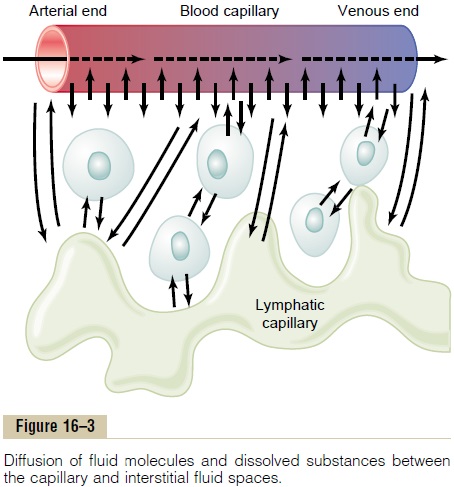
Diffusion results from thermal motion of the water molecules and dissolved substances in the fluid, thedifferent molecules and ions moving first in one direc-tion and then another, bouncing randomly in every direction.
Lipid-Soluble Substances Can Diffuse Directly Through the Cell Membranes of the Capillary Endothelium. If a substance islipid soluble, it can diffuse directly through the cell membranes of the capillary without having to go through the pores. Such substances include oxygen and carbon dioxide. Because these substances can per-meate all areas of the capillary membrane, their rates of transport through the capillary membrane are many times faster than the rates for lipid-insoluble substances, such as sodium ions and glucose that can go only through the pores.
Water-Soluble, Non-Lipid-Soluble Substances Diffuse Only Through Intercellular “Pores” in the Capillary Membrane.
Many substances needed by the tissues are soluble in water but cannot pass through the lipid membranes of the endothelial cells; such substances include watermolecules themselves, sodium ions, chloride ions, and glucose. Despite the fact that not more than 1/1000 ofthe surface area of the capillaries is represented by the intercellular clefts between the endothelial cells, the velocity of thermal molecular motion in the clefts is so great that even this small area is sufficient to allow tremendous diffusion of water and water-soluble sub-stances through these cleft-pores. To give one an idea of the rapidity with which these substances diffuse, therate at which water molecules diffuse through the cap-illary membrane is about 80 times as great as the rate at which plasma itself flows linearly along the capillary.That is, the water of the plasma is exchanged with the water of the interstitial fluid 80 times before the plasma can flow the entire distance through the capillary.
Effect of Molecular Size on Passage Through the Pores.
The width of the capillary intercellular cleft-pores, 6 to 7 nanometers, is about 20 times the diame-ter of the water molecule, which is the smallest molecule that normally passes through the capillary pores. Conversely, the diameters of plasma protein molecules are slightly greater than the width of the pores. Other substances, such as sodium ions, chloride ions, glucose, and urea, have intermediate diameters. Therefore, the permeability of the capillary pores for
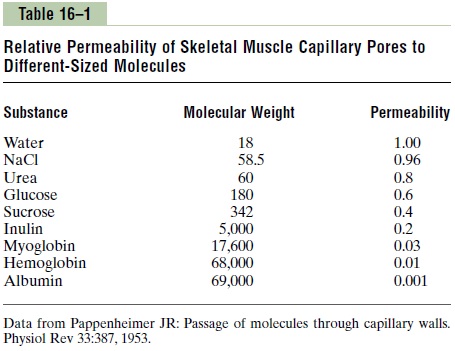
Table 16–1 gives the relative permeabilities of the capillary pores in skeletal muscle for substances com-monly encountered, demonstrating, for instance, that the permeability for glucose molecules is 0.6 times that for water molecules, whereas the permeability for albumin molecules is very, very slight, only 1/1000 that for water molecules.
A word of caution must be issued at this point. The capillaries in different tissues have extreme dif-ferences in their permeabilities. For instance, the mem-brane of the liver capillary sinusoids is so permeable that even plasma proteins pass freely through these walls, almost as easily as water and other substances. Also, the permeability of the renal glomerular mem-brane for water and electrolytes is about 500 times the permeability of the muscle capillaries, but this is not true for the plasma proteins; for these, the capillary permeabilities are very slight, as in other tissues and organs. When we study these different organs later in this text, it should become clear why some tissues—the liver, for instance—require greater degrees of capillary permeability than others to transfer tremendous amounts of nutrients between the blood and liver parenchymal cells, and the kidneys to allow filtration of large quantities of fluid for formation of urine.
Effect of Concentration Difference on Net Rate of Diffusion Through the Capillary Membrane. The “net” rate of diffu-sion of a substance through any membrane is propor-tional to the concentration difference of the substance between the two sides of the membrane. That is, the greater the difference between the concentrations of any given substance on the two sides of the capillary membrane, the greater the net movement of the sub-stance in one direction through the membrane. For instance, the concentration of oxygen in capillary blood is normally greater than in the interstitial fluid. Therefore, large quantities of oxygen normally move from the blood toward the tissues. Conversely, the con-centration of carbon dioxide is greater in the tissues than in the blood, which causes excess carbon dioxide
The rates of diffusion through the capillary membranes of most nutritionally important substances are so great that only slight concentration differences suffice to cause more than adequate transport between the plasma and interstitial fluid. For instance, the con-centration of oxygen in the interstitial fluid immedi-ately outside the capillary is no more than a few per cent less than its concentration in the plasma of the blood, yet this slight difference causes enough oxygen to move from the blood into the interstitial spaces to provide all the oxygen required for tissue metabolism, often as much as several liters of oxygen per minute during very active states of the body.
Related Topics