Matter Around Us | Term 1 Unit 3 | 7th Science - Elements | 7th Science : Term 1 Unit 3 : Matter Around Us
Chapter: 7th Science : Term 1 Unit 3 : Matter Around Us
Elements
Elements
Matter in its simplest form is called an element. We are using many elements in our daily life. The common salt is consisting of elements of Sodium and Chlorine. Water consists of Hydrogen and Oxygen. Magnesium and Phosphorus used for making crackers. Sulphur is used as manure in agriculture. Gallium is used for making mobile phones and silicon is used for making computer chips.
There are 118 known elements till date. 94 of these elements occur naturally while 24 elements have been created artificially in the laboratory.
Classification of Elements
We can classify the elements broadly intometals, non-metals and metalloids based upon their chemical properties.
The Robert Boyle is the first scientist used the term element. An early proponent of the elemental nature of matter and the nature of vacuum. He was known best for Boyle's Law.

Metals
We have tools, utensils and jewelry made from silver, copper, iron, gold, Aluminum. Using pressure like hammering or rolling we can deform these materials into various shapes. Such elements that are malleable (a material may be flattened into thin sheets or various shapes) is called as metals.
Metals are generally hard and shiny elements. Sodium is one of the exceptions as it is soft. All metals, except mercury are solids at room temperature.

Mercury is the only metal that is liquid at room temperature . Metals are malleable, can be bent or beaten into sheets. They can be drawn into wires. They are good conductors of heat and electricity. Copper, Lead, tin, nickel, iron, zinc, gold, magnesium and calcium are examples of metals.
Non-Metals
Non-metals are generally dull and soft. However, diamond is shiny and also the hardest natural substance on earth. Non-metals can be gases, solids, liquids. Non metals such as oxygen, hydrogen and chlorine are gases at room temperature. Non metals such as carbon, iodine, sulphur and phosphorus are solids at room temperature. Bromine is the only non-metal that is liquid at room temperature. Non-metals are poor conductors of heat and electricity. However, graphite (a form of the non-metal carbon) is a good conductor of electricity.

The difference between metals and non-metals
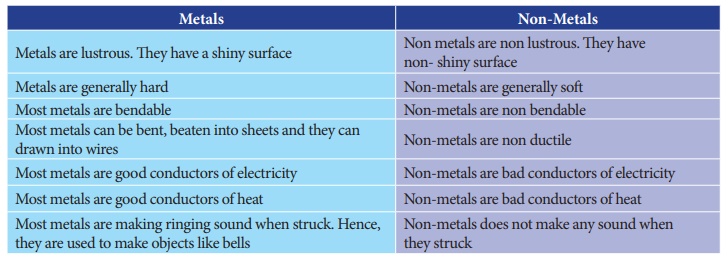
Metals
• Metals are lustrous. They have a shiny surface
• Metals are generally hard
• Most metals are bendable
• Most metals can be bent, beaten into sheets and they can drawn into wires
• Most metals are good conductors of electricity
• Most metals are good conductors of heat
• Most metals are making ringing sound when struck. Hence, they are used to make objects like bells
Non-metals
• Non metals are non lustrous. They have non- shiny surface
• Non-metals are generally soft
• Non-metals are non bendable
• Non-metals are non ductile
• Non-metals are bad conductors of electricity
• Non-metals are bad conductors of heat
• Non-metals does not make any sound when they struck
Metalloids
Metalloids exhibit the properties of bothmetals and non metals. Silicon, arsenic, antimony, and boron are some examples of metalloids.

ACTIVITY
Write down the symbols of the following elements.
Elements Symbol
Gold Au
Silver Ag
Copper Cu
Iron Fe
Nitrogen N2
Oxygen O2
Aluminium Al
Calcium Ca
Phosphorus P
Magnesium Mg
Potassium K
Sodium Na
Related Topics