Matter Around Us | Term 1 Unit 3 | 7th Science - Effect of temperature on Solid, Liquid and Gas | 7th Science : Term 1 Unit 3 : Matter Around Us
Chapter: 7th Science : Term 1 Unit 3 : Matter Around Us
Effect of temperature on Solid, Liquid and Gas
Effect of temperature on Solid, Liquid and Gas
What happens to matter during
heating?
The following are models of
particles in solids during heating. These models can be modified to represent
heating in Solids, Liquids and Gas.
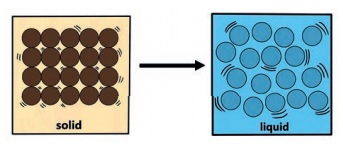
When solid is heated, the particles
gain energy and vibrate vigorously. The particles move slightly further apart
from one another.
This causes the volume of matter to
increase. This process is called expansion. How it is happens? The matter begun
to expand when heated. The volume increases due to the greater distance between
the particles. But the size of the particles remains in same size.
How do hot-air balloons float? When air inside the hot air
balloon is heated with a burner, it expands. The expansion causes the density
of the air inside the balloon to decrease. Hence, the air inside the balloon
has a lower density that the air outside of the balloon. This difference is
density allows the hot-air balloon to float.

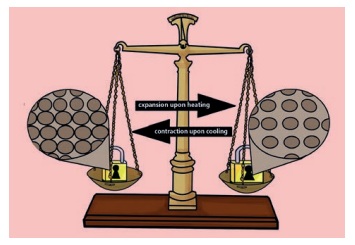
During heating or expansion, the
mass of matter does not change. This is explained in the following way. During
heating, the distance between the particles of the iron locks change. Mass is
conserved when matter expands.
Although the volume of the matter
changes, the size and number of the particles of matter do not change. Hence,
during heating, the mass of a matter is conserved. For example, in an iron lock
the distance between the iron particles increases when they gain enough heat.
However, the number of iron particles does not change. Hence the mass of the
iron lock is conserved.
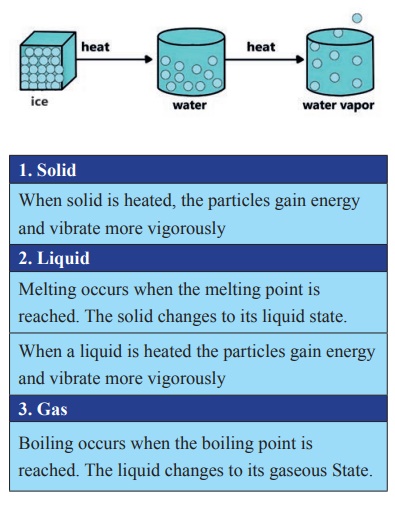
The melting of ice is an example of
a change in the states of matter. The change in the states of matter occurs
during melting, boiling and freezing and condensation.
When the particles possess enough
energy, they overcome the strong forces of attraction between one another. The
particles break free from one another and move randomly. For example, when
solid ice is heated to 00C, it melts to become liquids water. In the
same way, liquid water is heated to 1000C, it boils to become steam.
Related Topics