Matter Around Us | Term 1 Unit 3 | 7th Science - Compounds | 7th Science : Term 1 Unit 3 : Matter Around Us
Chapter: 7th Science : Term 1 Unit 3 : Matter Around Us
Compounds
Compounds
A compound is a pure substance that
is formed when the atoms of two or more elements combine chemically in definite
proportions.

Compounds exhibit properties
entirely different from the properties of their constituent elements. For
example, the atoms of the elements hydrogen and oxygen combine chemically in a
fixed ratio to form the compound water. However, water does not have the exact
same properties as hydrogen and oxygen. For example, at room temperature water
exist as liquid
while hydrogen and oxygen exist as gases. Also, oxygen supports fire whereas
water is used as a fire extinguisher.
Similarly, common salt (sodium chloride) is a compound made up of elements sodium and chlorine. It is used in our food, whereas sodium and chlorine are poison, are both unsafe for consumption.
ACTIVITY
Complete the following table by writing compounds of its
constituents.
Compound : Constituent Elements
Water : Hydrogen and Oxygen
Salt (Sodium chloride)
: Sodium and Chlorine
Sodium soda (sodium
bicarbonate) : Sodium, Carbon and Oxygen
Baking soda (sodium
bicarbonate) : Carbon, Hydrogen and
Oxygen
Sugar : Carbon, Hydrogen and Oxygen
Calcium oxide : Calcium and Oxygen
Calcium hydroxide : Calcium, hydrogen and Oxygen
Sodium hydroxide : Sodium, hydrogen and Oxygen
Potassium hydroxide : Potassium, hydrogen and Oxygen
Properties of Compounds
* A compound is formed only when the
constituent elements combine in a fixed proportion.
* The properties of a compound are
different from those of its constituent elements
* A compound can not be broken down
by physical methods. This is because acompound is made up of different elements
that are chemically combined. Sodium chloride cannot be separated by physical
methods such as filtration.
* A compound can be separated into its constituent elements by chemical methods only.
Difference between an element and
a compound
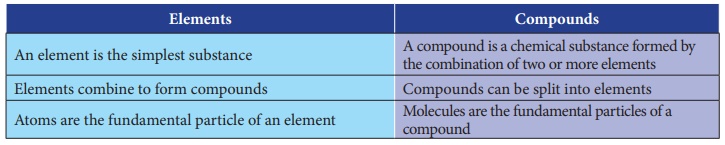
Elements
•
An element is the simplest substance
•
Elements combine to form compounds
•
Atoms are the fundamental particle of an element
Compounds
•
A compound is a chemical substance formed by the combination of two or more
elements
•
Compounds can be split into elements
•
Molecules are the fundamental particles of a compound
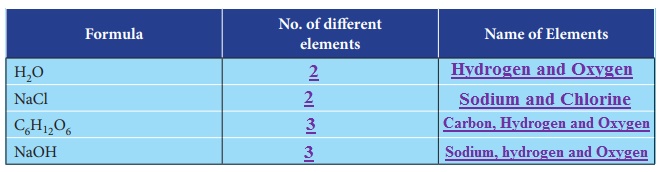
ACTIVITY
Complete the following table by counting the number of different elements in a compounds and give appropriate name.
Related Topics