Matter Around Us | Term 1 Unit 3 | 7th Science - Atomicity | 7th Science : Term 1 Unit 3 : Matter Around Us
Chapter: 7th Science : Term 1 Unit 3 : Matter Around Us
Atomicity
Atomicity
In chemistry we usually understand
atomicity to imply the total number of atoms present in one molecule of an
element, compound or a substance.
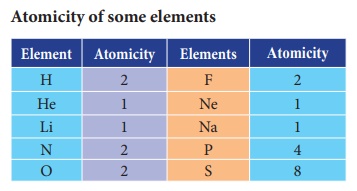
Let we see how to calculate the
atomicity of elements. For example, Oxygen exists as a diatomic molecule which
means that a molecule of oxygen contains two atoms hence its atomicity is 2.
O+O → O2
(Oxygen atom + Oxygen atom) → Oxygen Molecule
Similarly a phosphorus (P4)
molecule contains 4 atoms; a sulphur (S8) molecule contains 8
sulphur atoms. Hence their atomicity is 4 and 8 respectively.
For molecule containing more than
one types of atoms, simply count the number of each atom and that would be its
atomicity. For example, a molecule of sulphuric acid (H2SO4)
consists of 2 hydrogen atom, 1 sulphur atom and 4 oxygen atoms. Hence e its
atomicity is 2+1+4=7.
One molecule of water (H2O)
contains two atoms of hydrogen and one atom of oxygen, the atomicity of water
is three.
Elements in human Body
Nearly 99% of the mass of our human
body consists of just 6 chemical elements: oxygen, carbon, hydrogen, nitrogen,
calcium, andphosphorus. Another 5 elements make up most of the least percentage
point: potassium, sulphur, sodium, chlorine, and magnesium.
ACTIVITY
Write down atomicity of the following elements and
compounds.
Elements Atomicity
Cl 2
Na 1
K 1
Ca 1
Compounds Atomicity
H2O 3
Nacl 2
Elements in air
Air is a mixture of gases. The
molecules of two different elements, nitrogen and oxygen, make up about 99% of
the air. The rest includes small amounts of argon and carbon dioxide. (Other
gases such as neon, helium, and methane are present in trace amounts.) Oxygen
is the life-giving element in the air.
Related Topics