Chapter: Introduction to Human Nutrition: Nutrition and Metabolism of Lipids
Effect of diet on serum lipids and lipoproteins
Effect of diet on serum lipids and lipoproteins
Diet and serum cholesterol
Diet exerts a profound influence on blood lipids and lipoproteins and, as such, should always be a major component of strategies for the primary prevention of diseases in which lipids play an etiological role, such as CHD. Nevertheless, despite convincing epide-miological evidence and the existence of credible biochemical mechanisms to support a relationship between dietary fat and serum cholesterol, the outcome of prospective intervention trials designed to test this relationship within populations has been disappointing.
Over 30 years ago Keys and Hegsted made the land-mark observation that variation in the concentration of serum cholesterol across seven different countries was positively related to the amount of energy derived from saturated fat. Conversely, they found that intake of dietary PUFA was inversely related to serum cho-lesterol. From this finding they were able to formulate equations that enabled them to predict the quantita-tive effect of saturated and polyunsaturated fat on serum cholesterol (Figure 6.16). In simpler terms, the ratio of PUFAs to saturated fatty acids (SFAs), the P : S ratio, was used with effect to predict changes in serum cholesterol. Although still effective today, the equations and P:S ratio are being superseded by advanced knowledge of the biological effects of indi-vidual fatty acids. It is now well established that satu-rated fats with between 12 and 16 carbon atoms, namely lauric, myristic, and palmitic acids, are par-ticularly hypercholesterolemic, whereas stearic acid, an extremely abundant SFA in most diets, is relatively neutral in its effects on serum cholesterol. [Note that stearic acid is desaturated to monounsaturated fatty acids (MUFAs) by 9 desaturase]. The cholesterol-raising effect of SFAs arises chiefly from an increase in LDL cholesterol and is about twice as potent as the hypocholesterolemic effect of dietary PUFAs. Para-doxically, SFAs actually increase serum HDL choles-terol. The polyunsaturates are divisible into two main series on the basis of the position of the first double bond from the methyl end of the fatty acid chain, the parent fatty acids being linoleic (C18:2n-6) and α-linolenic (C18:3n-3) acids. The cholesterol-lowering effects of dietary PUFA is largely attributable to the effects of linoleic acid in lowering LDL. Historically,
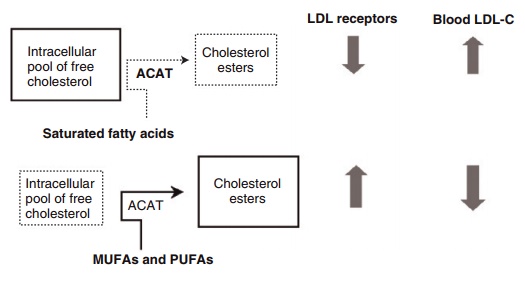
Figure 6.16 Influence of dietary fatty acids on serum cholesterol through differential effects on free cholesterol and low-density lipoprotein (LDL) receptor activity. ACAT, acyl-CoA-cholesterol acyl-transferase; LDL-C, LDL cholesterol; MUFA, monounsaturated fatty acid; PUFA, poly-unsaturated fatty acid.
monounsaturated fat was considered to be neutral with respect to its effects on lipids and lipoproteins and was omitted from the predictive formulae of Keys and Hegsted. However, further studies, prompted by interest in the role of MUFAs in the Mediterranean diet, have shown that MUFA-enriched diets may decrease LDL cholesterol, although possibly to a lesser extent than linoleic acid, and increase HDL choles-terol. An additional benefit of MUFAs is thought to be conferred by the presence of a single double bond in MUFAs, which when incorporated into the mem-brane phospholipids of LDL, protect this lipoprotein from oxidative modification, an essential prerequisite step in the deposition of cholesterol in the artery wall. In this regard, there has been concern that increasing dietary PUFA will impose additional oxidative stress on LDL. While this idea forms part of the rationale for limiting the amount of dietary PUFA to less than 10% of energy intake, there is as yet no convincing evidence of adverse effects from increasing the level of PUFA in tissues and circulating lipoproteins.
Trans fatty acids
While saturated fats consist of straight chains of carbon atoms which pack together tightly in the phospholipids of cell membranes and lipoproteins, in contrast, carbon double bonds in the cis configura-tion in MUFAs and PUFAs introduce a bend or kink into the carbon chain. This alters the physical proper-ties of the phospholipids containing these fatty acids, by, for example, increasing their packing volume, a physical property that contributes to an increase in membrane fluidity. The partial hydrogenation of MUFAs and PUFAs, most notably during the indus-trial processing of foods for the purpose of solidifying unsaturated oils, results in a loss of this kink as the fatty acid assumes a straighter, trans configuration that resembles that found in SFAs. This likeness in chemical structure is thought to account for the SFA-like effects of trans fatty acids such as elaidic acid (trans isomer of oleic acid) on serum lipids (see Figures 6.1 and 6.2). The results of prospective cohort studies such as the Nurses’ Health Study showed that high levels oftrans fats in excess of 7% energy increased serum LDLs and reduced HDLs (Willet et al. 1993). However, despite the continued use of par-tially hydrogenated fats in food products, the average intake of trans fatty acids in most Western diets does not exceed 2% of total energy intake, and at this level of intake these fats are unlikely to exert adverse effects on serum lipoproteins.
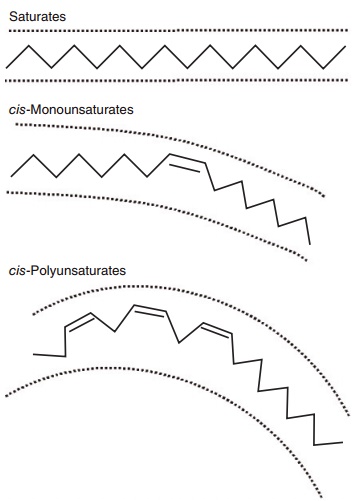
Figure 6.1 Stick models illustrating the basic structural differences between saturated, cis-monounsaturated, and cis-polyunsaturated fatty acids. As shown in two dimensions, the increasing curvature caused by inserting one or more double bonds increases the area occupied by the fatty acid. The physical area occupied by unsaturated fatty acids is further accentuated in three dimensions because esteri-fied fatty acids rotate around the anchored terminal.
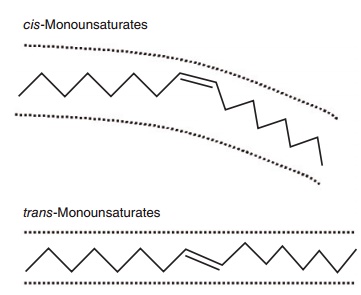
Figure 6.2 Stick models comparing a cis- with a trans-unsaturated fatty acid. A cis-unsaturated double bond creates a U-shaped space and confers curvature to the molecule because, relative to the longi-tudinal axis of the fatty acid, the two hydrogens at the double bond are on the same side of the molecule. A trans-unsaturated double bond does not confer curvature to the molecule because the hydrogens are on opposite sides of the double bond. A trans-double bond therefore tends to give the fatty acid physicochemical properties more like that of a saturated fatty acid.
Plant sterols and soluble nonstarch polysaccharides
These compounds may be grouped together as they share a similar mode of action on LDL cholesterol, which is to reduce the availability of dietary and biliary cholesterol for absorption in the gut. This action interrupts the enterohepatic circulation and upregulates the production and activity of LDL receptors. Plant sterols and their esters such as those incorporated into margarines (stanols and stanol esters), despite being nearly identical in structure to cholesterol, are poorly absorbed and interfere with the reabsorption of cholesterol origi-nating from bile (~1 g/day) and dietary sources (300 mg/day) by either coprecipitation or competi-tion. Margarines or spreads (30–40 g/day) containing plant sterols or their derivatives have been shown to reduce LDL cholesterol by up to 14% in controlled trials. Soluble NSPs such as those found in gums and gelling agents from fruit (gum arabic and pectins) act in a similar way and have been shown to be equally efficacious in reducing LDL cholesterol.
Dietary cholesterol
There is a popular misconception that dietary choles-terol correlates directly with serum cholesterol, when in fact dietary cholesterol, within a range of normal dietary consumption (100–400 mg/day), has only a very small impact on blood cholesterol levels. Eggs represent the principal source of dietary cholesterol in most diets (1 egg yolk = 150–250 mg cholesterol); in their absence, most Western diets would contain considerably less than 100 mg cholesterol/day. The classic but extreme egg-feeding studies showed that feeding of up to six eggs per day (900 mg cholesterol) increased LDL cholesterol acutely. However, the body effectively counters this effect with sensitive, compen-satory mechanisms to deal with an increasing load of dietary cholesterol, one of which is to reduce the amount of cholesterol absorbed in the gut. This com-pensation effectively abolishes any dose–response relationship between dietary cholesterol, over a prac-tically realistic range of intakes, and serum choles-terol. Two factors that may influence the variability in response to dietary cholesterol are dietary saturated fatty acids, which have been shown to augment the cholesterol-raising effects of dietary cholesterol, and a phenomenon of increased susceptibility to dietary cholesterol in some individuals for some, as yet, unknown reason.
To place these dietary influences on blood choles-terol in perspective with other cholesterol-lowering strategies, a metaanalysis of dietary intervention trials undertaken by the World Health Organization (WHO) revealed that dietary modification could achieve reductions in serum cholesterol of between only 4% and 5%. This finding is in sharp contrast to the potent effects of cholesterol-lowering drugs, which can reduce serum cholesterol by 30–40% and have been shown, unequivocally, to reduce the incidence of death from CHD. It also highlights the need to address other risk factors which are more responsive to dietary change.
Fat quantity versus quality: importance of the ratio of n-6:n-3 polyunsaturated fatty acids
The underlying principle for a reduction in total fat intake is to reduce energy intake from the consump-tion of the most energy-dense macronutrient in order to prevent weight gain and ultimately obesity. The current recommendation for the UK is to reduce energy derived from fat to 35% or less. Since weight gain is associated with raised plasma TAGs and abnor-malities in circulating lipoproteins, reducing total fat intake should, in theory, reduce blood lipids. However, in practice there is little evidence to support such an effect within populations. Metaanalyses have revealed that little benefit is to be gained, at least in terms of changes in blood lipids, by reducing total fat without altering the composition of dietary fatty acids. Metaanalyses have also helped to resolve the issue of what represents the most appropriate replacement nutrient for SFAs. Since PUFAs, and specifically lin-oleic acid, were shown to counter the actions of SFAs and were abundant in natural sources such as sun-flower and corn oils, they were an obvious first choice. The alternative was to substitute fat with dietary car-bohydrate. There have been problems associated with both of these approaches. First, increasing dietary lin-oleic acid excludes the lesser abundant, but more met-abolically active, n-3 PUFA, and especially the longer chain (C20–C22) members of this series derived from marine oils [C20:5 (eicosapentaenoic acid) and C22:6 (docosahexaenoic acid)]. Overemphasis on linoleic acid in the food industry, together with a widespread resistance to the consumption of fish, has increased the ratio of n-6 to n-3 PUFAs in northern Europe and the USA since the 1970s. This situation has major implications for the development of abnormalities in circulating lipoproteins, since deficiency in eicosapen-taenoic acid and docosahexaenoic acid could help to promote an increase in plasma TAG. This could occur through an overproduction of endogenous TAG (VLDL) in the liver and intolerance to dietary (exoge-nous) fat, and lead to the development of dyslipidemia known as an ALP. The frequency of this dyslipidemia is believed to be very rare in Mediterranean countries that have a high intake of dietary n-3 PUFA and an n-6:n-3 ratio closer to 1. High-carbohydrate diets have been shown to increase plasma TAG. Carbohydrate-induced hypertriacylglyc-erolemia is not, as was originally thought, a short-term adaptive response in the liver, as it changes its pattern of oxidation from fat to carbohydrate, but a real phenomenon associated with the overconsump-tion of the non-milk extrinsic sugars, sucrose and fructose, most notably in individuals with insulin-resistant dyslipidemia. There is evidence to suggest that this effect can be avoided by limiting the intake of sucrose and increasing the intake of slowly absorbed carbohydrate with a low impact on blood glucose.
The results of several metaanalyses of dietary inter-vention trials support dietary MUFAs as the most favored substitute for dietary saturated fatty acids, and even linoleic acid in areas of high intake.
Effects of n-3 polyunsaturated fatty acids from plants and fish
The current dietary recommendation for the intake of long-chain n-3 PUFAs (eicosapentaenoic acid/doc-osahexaenoic acid) in the UK is 450 mg/day (SACN, 2004). This was to increase intake by consuming two portions of fish per week, one of which should be oily (e.g., mackerel, sardines). This recommendation was based on evidence from a host of epidemiological and intervention studies, which showed that regular fish consumption could reduce the risk of sudden cardiac death. Since this an acute end-point of CHD, the ben-efits of fish oil have been ascribed to the prevention of fatal cardiac arrhythmia, and, to a lesser extent, coronary thrombosis, but not to any favorable effects on blood lipids. However, there is also convincing evidence to show that fish oil supplementation (1 g/ day for 3 years) reduces the incidence of death from CHD in healthy, free-living subjects. This longer term benefit may be linked to the effects of eicosapentae-noic acid/docosahexaenoic acid on a host of other cardiovascular risk factors, including plasma TAGs and lipoproteins.
Long-chain n-3 PUFAs exert multiple effects on lipid metabolism, the most notable of which is the capacity to decrease postabsorptive plasma TAG levels by 20–30%. Fish oil-enriched diets have also been shown to attenuate the magnitude and duration of postprandial lipemia following the ingestion of a fat-containing meal. These effects are frequently accom-panied by beneficial changes in circulating LDLs and HDLs, and the correction of an ALP.
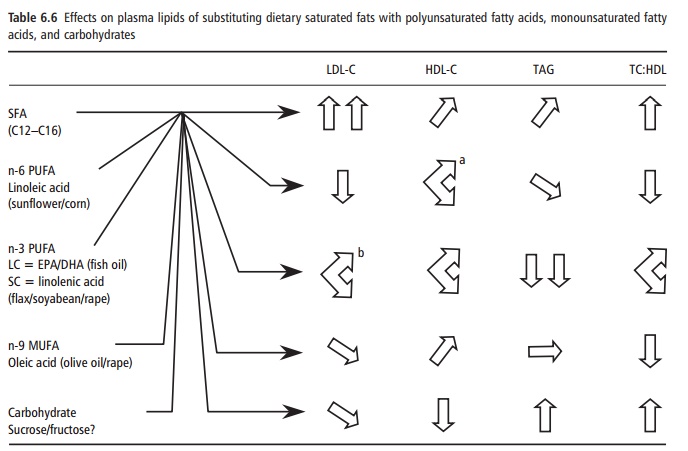
Widespread knowledge of the favorable effects of eicosapentaenoic acid and docosahexaenoic acid has raised awareness of the need to increase intakes of these fatty acids, and to reduce the amount of n-6 PUFA at the same time. However, in practice, this will be difficult to achieve, not least because of a mass resistance to the increased consumption of oily fish and diminishing fish stocks. An obvious alternative would be to increase the intake of the shorter chain precursor of eicosapentaenoic acid/docosahexaenoic acid, α-linolenic acid (C18:3n-3). The latter is derived from plant seeds such as flax and rapeseed, and is desaturated and elongated to its longer chain relatives in the body. Unfortunately, the rate of conversion to eicosapentaenoic acid and especially docosahexaenoic acid is slow, and the efficiency of conversion is reduced by high levels of linoleic acid, which competes more effectively than α-linolenic acid for desaturation. There is, as yet, no evidence to suggest that the rate of conversion of dietary α-linolenic acid to eicosa-pentaenoic acid and especially docosahexaenoic acid is sufficient to achieve fish oil-like effects on blood lipids (Table 6.6).
How do dietary fatty acids influence serum cholesterol and triacylglycerols?
In common with other physiological systems, lipo-protein metabolism is coordinated by interplay between the activity of specific genes and hormones that determines the production and activity of func-tional proteins (enzymes, receptors, lipid transfer, and apoproteins). Effects on these functional proteins ultimately regulate the quantity and quality of circu-lating lipids and lipoproteins. While there have been significant advances in knowledge of the modulatory effects of dietary fatty acids on hormones and gene expression, evidence for the effects of dietary fats on functional proteins is by far the most advanced.
Saturated fatty acids and low-density lipoprotein cholesterol
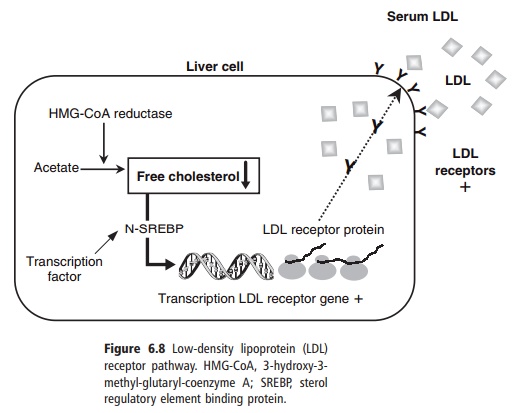
The most well-elucidated mechanism to explain how different dietary fats produce variable effects on LDL-cholesterol is through the LDL receptor pathway, the control of which has already been described. The ability of the cell to regulate its pool of free cholesterol depends to a large extent on the nature of the fatty acids available for esterification by the enzyme ACAT, an intracellular relative of LCAT. ACAT favors unsaturated fatty acids (MUFAs and PUFAs) as substrates for esterification, which utilizes free cholesterol within the cell. The resulting reduc-tion in intracellular free cholesterol stimulates the transcription of the LDL receptor gene and produc-tion of new LDL receptors through the SREBP mech-anism, and fall in circulating LDL as already described. Conversely, SFAs are poor substrates for ACAT, and their presence in the cell exerts the opposite effect on free cholesterol levels, thus increasing circulating LDL cholesterol and total serum cholesterol (Figure 6.8). Fatty acids may also exert direct effects on the activity of LDL receptors by altering the composition of membrane phospholipids and thus membrane fluid-ity. Alternatively, there is evidence to suggest that dietary PUFA could upregulate LDL receptors indi-rectly, by increasing the cholesterol content (litho-genicity) of bile and in this way accelerate the excre-tion of cholesterol.
Long-chain n-3 polyunsaturated fatty acids and serum triacylglycerols
Long chain n-3 PUFAs have potent effects in the liver, where they suppress the production of endogenous TAG by inhibiting the enzymes phosphatidic acid phosphatase and diacylglycerol acyltransferase. They may also selectively increase the degradation of apoB-100, further reducing the production of TAG-rich VLDLs. (Note that apoB-100 is produced constitu-tively, so that the production of VLDL is driven by the supply of substrates for the synthesis of TAG). In addition, long-chain n-3 PUFAs accelerate the clear-ance of TAG-rich lipoproteins from the circulation in the postprandial phase by stimulating the activity of LPL. Together, these effects are thought to underlie the ability of these fatty acids to correct the lipopro-tein abnormalities associated with an ALP. It is also possible that many of the effects of eicosapentaenoic acid/docosahexaenoic acid on blood lipids and other cardiovascular risk factors are mediated through an increase in the sensitivity of tissues to the action of insulin. However, there is, as yet, no convincing evi-dence to support such an effect in adipose tissue, liver, or skeletal muscle.
Nutrient–gene interactions
It has been estimated that diet could account for up to 50% of the variation in blood lipids and lipopro-tein levels between individuals. This would mean that genetic differences must explain the remaining 50%. In real terms, interactions between diet and genes represent a sizeable proportion of each of these unre-alistically discrete fractions.
Fixed genetic polymorphisms
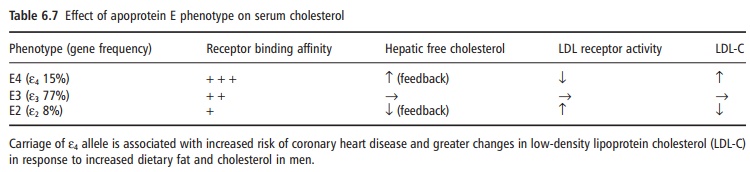
Variation in the structure of specific genes between individuals (genetic heterogeneity) has been shown to give rise to differences in dietary responsiveness. A few common polymorphisms have been identified in genes associated with lipoprotein metabolism, the best example of which is apoE. ApoE facilitates the uptake of TAG-rich lipoproteins (chylomicron rem-nants and VLDL) via the remnant and LDL receptors and, thus, in part, determines the removal of TAG from the circulation. The gene for apoE is polymor-phic, which means that it exists in multiple forms between individuals. This polymorphism generates several isoforms of the protein that express variable affinities for their receptors and thus variable poten-tial to remove TAG-rich lipoproteins from the circu-lation. In this way, apoE genotype can modulate the response of an individual to any dietary fat that exerts an influence on TAG-rich lipoproteins, giving rise to
Modulation of gene expression
Dietary fatty acids and/or their intracellular (eico-sanoid) derivatives can also influence the expression of genes (rate of gene transcription) by interacting with specific nuclear receptors within the nucleus of cells. These nuclear receptors control the rate of gene transcription by binding to specific regions of DNA known as responsive elements. Genes associated with the production of functional proteins can be either stimulated or repressed according to the nature of the nuclear transcription factor and its binding substrate (PUFAs or derivative). Peroxisome proliferator-acti-vated receptors (PPARs) represent examples of nuclear receptors that may utilize long-chain PUFAs as sub-strates. PPARs can be found in all tissues of the body, but notably in the liver, where they control the syn-thesis of lipid and apoproteins (PPAR-α), and in adipose tissue (PPAR-γ), where they control the dif-ferentiation of adipocytes and insulin-sensitive mobi-lization and synthesis of TAG. SREBPs represent another example of nuclear transcription proteins that control cholesterol and fatty acid metabolism within the cell.
Related Topics