Chapter: Introduction to Human Nutrition: Nutrition and Metabolism of Lipids
Long-chain fatty acid metabolism
Long-chain fatty acid metabolism
Synthesis
Synthesis of fatty acids occurs in the cytosol. It begins with
acetyl-CoA being converted to malonyl-CoA by acetyl-CoA carboxylase, an enzyme
dependent on biotin. Malonyl-CoA and a second acetyl-CoA then condense via β-ketothiolase. This is subsequently reduced, dehydrated, and
then hydrogenated to yield a four-carbon product that recycles through the same
series of steps until the most common long-chain fatty acid product, palmitate,
is produced (Figure 6.10). Acetyl-CoA is primarily an intramitochondrial
product. Thus, the transfer of acetyl-CoA to the cytosol for fatty acid
synthesis appears to require its conversion to citrate to exit the mitochondria
before being reconverted to acetyl-CoA in the cytosol.
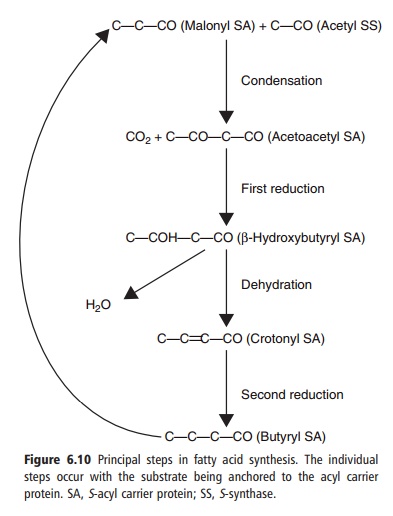
Figure 6.10 Principal steps in fatty acid synthesis. The individual steps occur with the substrate being anchored to the acyl carrier protein. SA, S-acyl carrier protein; SS, S-synthase.
There are three main features of long-chain fatty acid synthesis
in mammals:
1 inhibition by starvation
2 stimulation by feeding carbohydrate after fasting
3 general inhibition by dietary fat.
Carbohydrate is an important source of carbon for generating
acetyl-CoA and citrate used in fatty acid synthesis. Enzymes of carbohydrate
metabolism also help to generate the NADPH needed in fatty acid synthesis.
Acetyl-CoA carboxylase is a key control point in the pathway and is both
activated and induced to polymerize by citrate. Acetyl-CoA carboxylase is
This is probably one important negative feedback mechanism by which
both starva-tion and dietary fat decrease fatty acid synthesis. High amounts of
free long-chain fatty acids would also compete for CoA, leading to their β-oxidation. Elongation of palmitate to stearate, etc., can occur
in mitochondria using acetyl-CoA, but is more com-monly associated with the
endoplasmic reticulum where malonyl-CoA is the substrate.
Humans consuming >25% dietary fat synthesize relatively
low amounts of fat (<2 g/day). Compared with other animals, humans also appear to
have a relatively low capacity to convert stearate to oleate and linoleate or α-linolenate to the respective longer chain polyunsaturates.
Hence, the fatty acid profiles of most human tissues generally reflect the
intake of dietary fatty acids; when long-chain n-3 PUFAs are present in the
diet, this is evident in both free-living humans as well as in experimental
animals. Neverthe-less, fatty acid synthesis is stimulated by fasting/
refeeding or weight cycling, so these perturbations in normal food intake can
markedly alter tissue fatty acid profiles.
Oxidation
β-Oxidation is the process by which fatty acids are utilized for energy. Saturated fatty acids destined for β-oxidation are transported as CoA esters to the outer leaflet of mitochondria by FABP. They are then translocated
inside the mitochondria by carnitine acyl-transferases. The β-oxidation process involves repeated dehydrogenation at
sequential two-carbon steps and reduction of the associated flavoproteins
(Figure 6.11). Five ATP molecules are produced during production of each
acetyl-CoA. A further 12 ATP molecules are produced after the acetyl-CoA
condenses with oxaloacetate to form citrate and goes through the tricarboxylic
acid cycle.
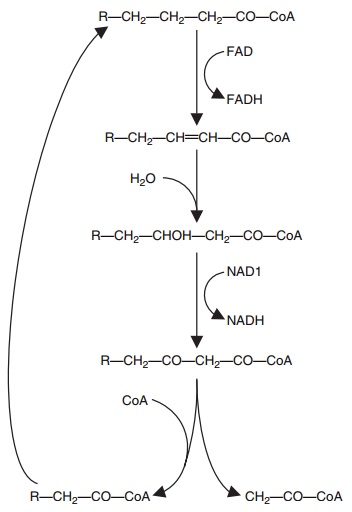
Figure 6.11 Principal steps in β-oxidation of a saturated fatty acid. The steps shown follow fatty acid “activation” (binding to coenzyme A) and carnitine-dependent transport to the inner surface of the mito-chondria. Unsaturated fatty acids require additional steps to remove the double bonds before continuing with the pathway shown. FAD, flavin adenine dinucleotide; FADH reduced flavin adenine dinucleo-tide; R, 12 carbons.
The efficiency of fatty acid oxidation depends on the
availability of oxaloacetate and, hence, concurrent carbohydrate oxidation. β-Oxidation of saturated fatty acids appears to be simpler than
oxidation of unsaturated fatty acids because, before the acetyl-CoA cleavage,
it involves the formation of a trans
double bond two carbons from the CoA. In contrast, β-oxidation of unsaturated fatty acids yields a double bond in a different position that then requires further
isomerization or hydrogenation. From a biochemical perspective, this extra step
appears to make the oxida-tion of unsaturated fatty acids less efficient than
that of saturated fatty acids. However, abundant in vivo and in vitro
research in both humans and animals clearly shows that long-chain cis-unsaturated fatty acids with one to
three double bonds (oleate, linole-ate, α-linolenate) are more readily β-oxidized than saturated fatty acids of equivalent chain length,
such as palmitate and stearate. The oxidation of PUFA and monounsaturates in
preference to saturates has potential implications for chronic diseases such as
coronary artery disease because their slower oxida-tion implies slower
clearance from the blood, thereby providing more opportunity for esterification
to cho-lesterol and subsequent deposition in the vessel wall.
Odd-carbon long-chain fatty acids are relatively uncommon but,
when β-oxidized, yield propionylCoA, the further β-oxidation of which requires biotin and vitamin B12
as coenzymes.
Ketogenesis and ketosis
Large amounts of free fatty acids inhibit glycolysis and the
enzymes of the tricarboxylic acid cycle, thereby impairing production of
oxaloacetate. When insufficient oxaloacetate is available to support the
continued oxidation of acetyl-CoA, two acetyl-CoA molecules condense to form a
ketone, acetoacetate. Acetoacetate can be spontaneously decarboxylated to form
acetone, a volatile ketone, or converted to a third ketone, β-hydroxybutyrate. When glucose is limiting, ketones are an
alternative source of energy for certain organs, particularly the brain. They
are also efficient substrates for lipid synthesis during early postnatal
development. Conditions favoring ketogenesis include starvation, diabetes, and
a very high-fat, low-carbohydrate “ketogenic” diet.
Carbon recycling
Carbon recycling is the process by which acetyl-CoA derived from
β-oxidation of one fatty acid is incor-porated into another lipid
instead of completing the β-oxidation process to carbon dioxide.
In principle, all fatty acids undergo this process to some extent but it is
most clearly evident for two PUFAs, linoleate and α-linolenate. Carbon recycling captures the over-whelming
majority of α-linolenate carbon, i.e., about 10 times more than is
incorporated into docosahexae-noate, which remains in the body of suckling rats
48 hours after dosing with uniformly 13C-labeled α-lino-lenate. Carbon recycling of linoleate in the rat cap-tures
similar amounts of the linoleate skeleton to those of arachidonate, the main
desaturation and chain-elongation product of linoleate. Hence, carbon recycling
appears to be a ubiquitous feature of the metabolism of PUFA, although its
biological signifi-cance is still unclear.
Peroxidation
Peroxidation (auto-oxidation) is the nonenzyme-cat-alyzed
reaction of molecular oxygen with organic compounds to form peroxides and
related breakdown products. PUFAs are particularly vulnerable to peroxi-dation
at the double bonds. Initiating agents such as pre-existing peroxides,
transition metals, or ultraviolet or ionizing radiation produce singlet oxygen.
Singlet oxygen can then abstract hydrogen at the double bonds of
polyunsaturates to produce free (peroxy) radicals, which abstract further hydrogens
from the same or different fatty acids and propagate the peroxidation process.
Eventually, this leads to termination by the formation of stable degradation
products or hydro-peroxides (Figure 6.12). Trans
isomers are frequently formed during the process. Hydroperoxides can form
further hydroperoxy radicals or can be reduced by antioxidants, which contain
thiol groups, i.e., glutathi-one and cysteine. Peroxidation of dietary fats
gives rise to aldehydes, i.e., 2-undecenal, 2-decenal, nonanal, or octanal,
which have a particular odor commonly known as rancidity.

Figure 6.12 Principal steps in peroxidation of a polyunsaturated fatty acid.
Since peroxidation is a feature of polyunsaturates, it is a potential hazard facing most membranes and dietary lipids. Antioxidants such as vitamin E are usually present in sufficient amounts to prevent or block peroxidation in living tissues. Humans and animals readily detect peroxidized fats in foods by their disagreeable odor and avoid them. However, modeling the effects of peroxides produced in vivo and in vitro is particularly challenging because lipid peroxidation undoubtedly is an important part of several necessary biological processes such as activa-tion of the immune response.
Desaturation, chain elongation, and chain shortening
One
important characteristic of long-chain fatty acid metabolism in both plants and
animals is the capacity to convert one to another via the processes of
desatu-ration, chain elongation, and chain shortening.
Plants
and animals use desaturases to insert a double bond into long-chain fatty
acids. There are several desaturases, depending on the position in the acyl
chain into which the double bond is inserted. Although myristate (14:0) and
palmitate can be converted to their monounsaturated derivatives, myristoleate
(14:1n-5) and palmitoleate (16:1n-7) respectively, commonly it is only the
fatty acids of 18 or more carbons that undergo desaturation. The 9 desaturases
in all organisms, except for anaerobic bac-teria, use oxygen and NADPH to
introduce a cis double bond at carbons 9 and 10 of stearate. This is
accomplished by an enzyme complex consisting of a series of two cytochromes and
the terminal desatu-rase itself. The acyl-CoA form of fatty acids is the usual
substrate for the desaturases, but fatty acids esterified to phospholipids can
also be desaturated in situ.
All
mammals that have been studied can convert stearate to oleate via 9 desaturase.
However, in the absence of dietary oleate, young rats may have insuf-ficient
capacity to sustain normal tissue oleate levels. Normal values depend on the
reference, which can vary widely depending on the source and amount of oleate
in the diet. Nevertheless, it is important to dis-tinguish between the
existence of a given desaturase and the capacity of that pathway to make
sufficient of the necessary product fatty acid. Hence, as with the long-chain
polyunsaturates and, indeed, with other nutrients such as amino acids, it is
important to keep in mind that the existence of a pathway to make a particular
fatty acid or amino acid does not guarantee sufficient capacity of that pathway
to make that product. This is the origin of the concept of “conditional
essentiality” or “indispensability.” Both plants and animals are capable of
desaturating at the 9–10 carbon ( 9 desaturase) of stearate, result-ing in
oleate. However, only plants are capable of desaturating oleate to linoleate
and then to α-lino-lenate. Once linoleate and α-linolenate are consumed by
animals, their conversion to the longer chain PUFAs of their respective
families proceeds primarily by an alternating series of desaturation ( 6 and 5
desaturases) and chain-elongation steps (Figure 6.13). Sequential desaturations
or chain elongations are also a possibility, resulting in a large variety,
though low abundance, of other PUFAs.
During
dietary deficiency of linoleate or α-lino-lenate, oleate can also be
desaturated and chain elon-gated to the PUFA eicosatrienoate (20:3n-9). Hence,
most but not all PUFAs are derived from linoleate or α-linolenate.
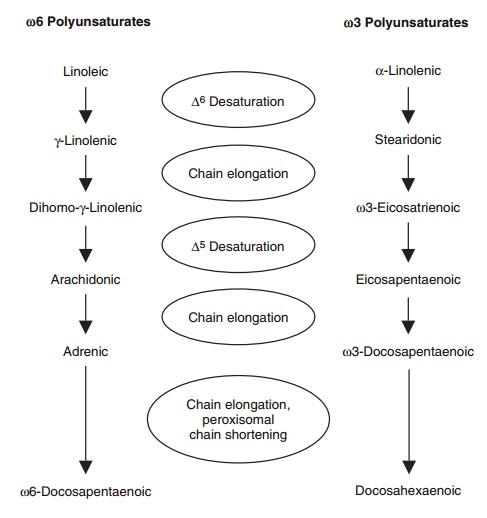
Figure 6.13 Conversion of linoleic (18:2n-6) and α-linolenic (18:3n-3) acids to their respective longer chain, more
unsaturated polyunsaturates. In mem-branes, linoleic and arachidonic acids are
the prin-cipal n-6 polyunsaturates, while docosahexaenoic acid is the principal
n-3 polyunsaturate. Hence, these two families of fatty acids have different
affinities for the desaturation and chain-elongation enzymes. This pathway is
principally based in the endoplasmic reticulum but appears to depend on
peroxisomes for the final chain shortening, which involves 24 carbon
intermediates that are not illustrated.
Chain elongation of saturated and unsaturated fatty acids occurs
primarily in the endoplasmic retic-ulum, although it has also been demonstrated
to occur in mitochondria. Unlike the desaturation steps immediately before and
after, the elongation steps do not appear to be rate limiting in the metabolism
of linoleate or α-linolenate.
Despite the capacity to insert at least three double bonds in
both n-3 and n-6 polyunsaturates, there is no proof that a 4 desaturase
exists to insert the final double bond in docosapentaenoate (22:5n-6) or
doc-osahexaenoate (Figure 6.13). Rather, it appears that the precursors to
these two fatty acids undergo a second elongation, repeated 6
desaturation followed by chain shortening in peroxisomes. This unexpect-edly
convoluted series of steps is corroborated by the docosahexaenoate deficiency
observed in disorders of peroxisomal biogenesis such as Zellweger’s syndrome.
Hydrogenation
Opposite to the desaturation process is hydrogena-tion or removal of unsaturated bonds in lipids. Rumen bacteria are the only organisms known to have this capability.
As in chemical hydrogenation practiced by the food industry, biohydrogenation
in the rumen can be incomplete, resulting in the formation of small amounts of trans isomers, particularly of oleate,
lino-leate, and α-linolenate, which are found in milk fat.
Eicosanoids
Eicosanoids are 20-carbon, oxygen-substituted cyclized
metabolites of dihomo-γ-linolenate, arachi-donate, or
eicosapentaenoate. They are produced via a cascade of steps starting with the
cyclooxygenase or lipoxygenase enzymes present in microsomes. The main
cyclooxygenase products comprise the classical prostaglandins, prostacyclin and
the thromboxanes. The main lipoxygenase products are the leukotrienes
(slow-reacting substances of anaphylaxis) and the noncyclized hydroperoxy
derivatives of arachidonate that give rise to the hepoxylins and lipoxins
(Figure 6.14).
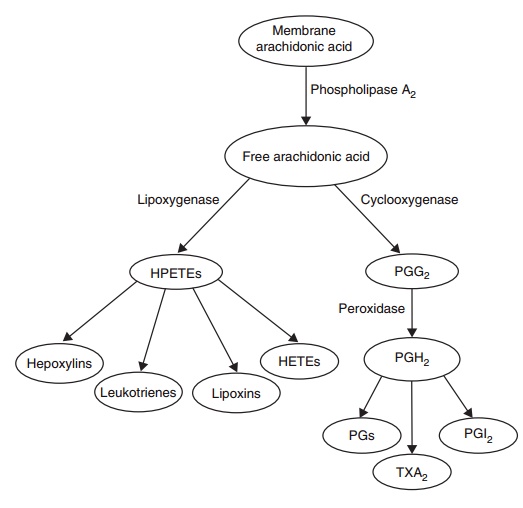
Figure 6.14 The
arachidonic acid cascade is a
fundamental component of cell signal-ing during injury. Phospholipase A2 is immediately activated and the
free arachi-donic acid thus released is accessible to a controlled peroxidation
process involving several cyclooxygenases (constitutive or inducible) and
lipoxygenases. Over 50 metabolically active products are poten-tially produced,
depending on the tissue involved, the type of cell that has been stimulated,
and the type of injury. Only the main classes of these metabolites are shown.
Before excretion, they are further metabolized to stable products that are not
shown. Several of the cyclooxygenase products are competitive with each other,
such as the platelet-aggregating and blood vessel wall-constricting effects of
thromboxane A2 (TXA2) produced in plate-lets, versus
the opposite effects of prosta-cyclin (PGI2) derived from the blood vessel
wall. HETE, hydroxyeicosatetraenoic acid; HPETE, hydroperoxyeicosatetraenoic
acid; PG, prostaglandin; TX, thromboxane.
usually in response to an injury or a stimulus that releases the
free precursor, most commonly arachido-nate. The site of highest eicosanoid
concentration appears to be the seminal fluid, although some species have no
detectable eicosanoids in semen. Eicosanoids are second messengers modulating,
among other pathways, protein phosphorylation. The lung is a major site of
eicosanoid inactivation.
Four important characteristics of eicosanoid action should be
noted. First, individual eicosanoids often have biphasic actions as one moves
from very low through to higher, often pharmacological, concentra-tions. Thus,
effects can vary dramatically depending not only on the experimental system but
also on the eicosanoid concentration used. Second, several of the more abundant
eicosanoids arising from the same precursor fatty acid have opposite actions to
each other. For instance, prostacyclin and thromboxane A2 are both
derived from arachidonate but the former originates primarily from the
endothelium and inhibits platelet aggregation, while the latter orig-inates
primarily from platelets and is a potent plate-let-aggregating agent. Third,
competing eicosanoids derived from dihomo-γ-linolenate (1 series) and from eicosapentaenoate (3 series)
often have effects that oppose those derived from arachidonate (2 series)
(Figures 6.14 and 6.15). Thus, unlike prostaglandin E2,
prostaglandin E1 has anti-inflammatory actions, reduces vascular
tone, and inhibits platelet aggrega-tion. Fourth, varying the ratio of the
precursor fatty acids in the diet is an effective way to modify eico-sanoid
production. Thus, eicosapentaenoate and dihomo-γ-linolenate inhibit the synthesis of 2 series eicosanoids
derived from arachidonate. This occurs by inhibiting arachidonate release from
membranes by phospholipase A2 and its cascade through the
cyclooxygenases and lipoxygenases. The overproduc-tion of 2 series eicosanoids
is associated with higher blood pressure, increased platelet aggregation, and
inflammatory processes, and can be effectively inhib-ited by dietary approaches
using oils rich in eicosap-entaenoate and γ-linolenate (18:3n-6), the precursor to dihomo-γ-linolenate.
Stable analogues of some classical prostaglandins have
specialized medical applications, including the termination of pregnancy and
the closing of a patent
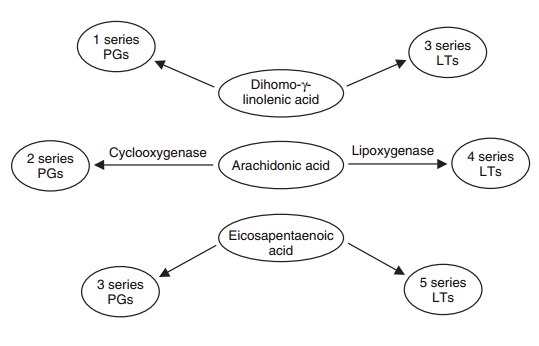
Figure 6.15 Arachidonic acid is not the only 20-carbon polyunsaturated fatty acid that can be
metabolized via the cyclooxygenases and lipoxygen-ases; both dihomo-γ-linolenic
acid (20:3n-6) and eicosapentaenoic acid (20:5n-3) are well-established
precur-sors as well, and produce prostaglan-dins (PGs) and leukotrienes (LTs)
that are frequently competitive with those produced from arachidonate, thereby
neutralizing the effects of the arachido-nate cascade (see Figure 6.14). This
provides a critical balance in the overall reaction to cell injury.
ductus arteriosus shortly after
birth. Many anti-inflam-matory and anti-pyretic drugs are inhibitors of
eico-sanoid synthesis. One potentially dangerous side-effect of inhibiting
eicosanoid synthesis is gastric erosion and bleeding. Receptor antagonists of
leukotrienes are effective in reducing the symptoms of asthma.
Related Topics