Chapter: Biotechnology Applying the Genetic Revolution: Immune Technology
Diabodies and Bispecific Antibody Constructs
DIABODIES
AND BISPECIFIC ANTIBODY CONSTRUCTS
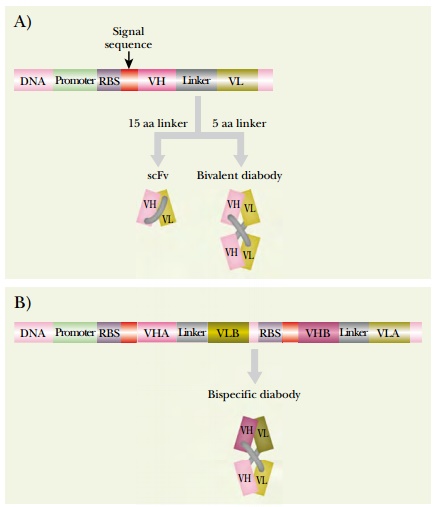
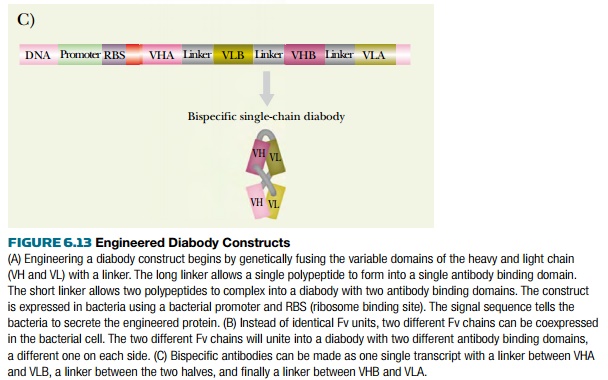
A variety of engineered antibody constructs are presently being investigated. A diabody consists of two single-chain Fv (scFv) fragments assembled together. Shortening the linker from 15 amino acids to five drives dimerization of two scFv chains. This no longer allows intrachain assembly of the linked VH and VL regions. The dimer consists of two scFv fragments arranged in a crisscross manner (Fig. 6.13). The resulting diabody has two antigen binding sites pointing in opposite directions. If two different scFv fragments are used, the result is a bispecific diabody that will bind to two different target proteins simultaneously. Note that formation of such a bispecific diabody requires that VH-A be linked to VL-B and VH-B to VL-A. It is of course possible to engineer both sets of VH and VL regions onto a single polypeptide chain encoded by a single recombinant gene, as shown in Fig. 6.13. Bispecific diabodies have a variety of potential uses in therapy, because they may be used to bring together any two other molecules; for example, they might be used to target toxins to cancer cells.
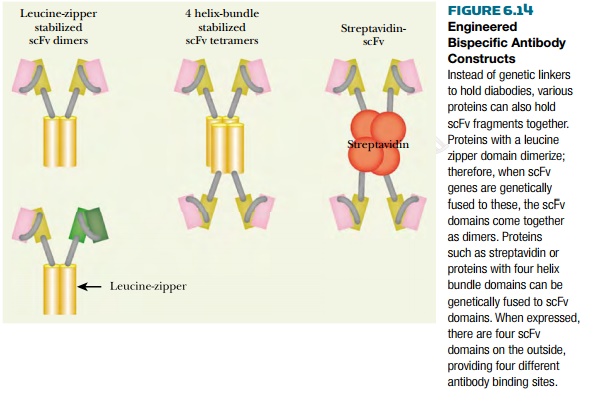
Another way to construct an
engineered bispecific antibody is to connect the two different scFv fragments
to other proteins that bind together (Fig. 6.14). Two popular choices are
streptavidin and leucine zippers. Streptavidin is a small biotin binding
protein from the bacterium
Streptococcus. It forms tetramers, so it allows up to four antibody fragments
to be assembled together. Furthermore, binding
to a biotin column can purify the final constructs. Leucine zipper
regions are used by many transcription factors that form dimers. Often, such
proteins form mixed dimers when their leucine zippers recognize each other and
bind together. Leucine zipper regions from two different transcription factors
that associate (e.g., the Fos and Jun proteins) may therefore be used to
assemble two different scFv fragments.
Related Topics