Chapter: Biotechnology Applying the Genetic Revolution: Noninfectious Diseases
Cyclic AMP as Second Messenger
CYCLIC
AMP AS SECOND MESSENGER
The regulatory nucleotide cyclic AMP (cAMP) is often used as a second
messenger by multicellular animals. Comparison of the way in which cyclic AMP
is used by bacteria, single-celled eukaryotes, and higher organisms illustrates
how the interplay among signaling, multicellularity, and reproduction has
developed as we ascend the evolutionary ladder.
Cyclic AMP is synthesized from ATP by
the enzyme adenylate cyclase, which
is located in the cytoplasmic membranes of both bacteria and eukaryotes. Cyclic
AMP is widespread in nature, but its precise regulatory function varies widely.
In bacteria cAMP often regulates gene expression in response to nutrient
availability, although the nutrient may vary from one type of bacteria to
another. In animals, cAMP is often used as a second messenger, transmitting a signal
from a receptor to the nucleus.
Despite the apparent differences, there
are fundamental similarities between the prokaryotic and eukaryotic signaling
systems. The level of cAMP depends on the activity of adenylate cyclase, which
in turn depends on signals received from other proteins. In both bacteria and animals,
this original signal originates from outside the cell via a membrane protein. In
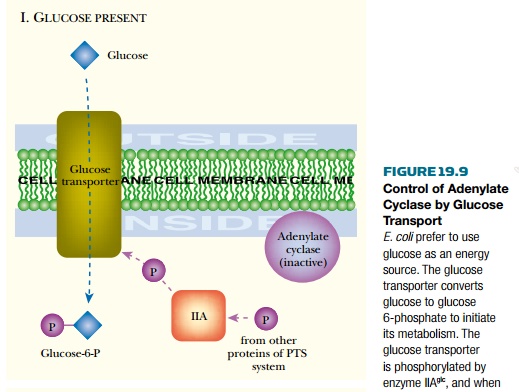
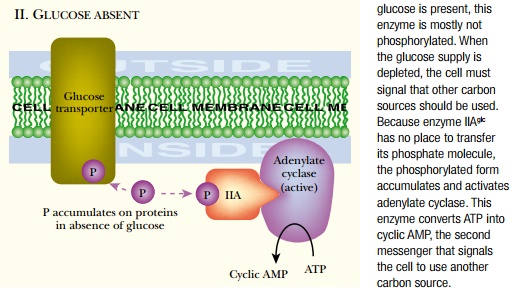
the case of Escherichia coli , adenylate cyclase responds to the
external presence of glucose (or other highly favored sugars). The proteins of
the phosphotransferase system (PTS) , which transports glucose and other
sugars, transmit the signal to adenylate cyclase. As glucose enters the
bacterial cell, it receives a phosphate group from the glucose transporter and
is converted to glucose 6-phosphate. When glucose is plentiful, it consumes so
manyphosphate groups that the proteinsof the PTS are mostly in
theirnonphosphorylated state ( Fig.19.9 ). When glucose is scarce, the phosphorylated
forms accumulate. In particular, phosphorylated enzyme IIA glc is involved in
signal transmission. This binds to and activates adenylate cyclase to make cyclic
AMP.
In animals, adenylate cyclase often
responds to hormonal messages that reveal the nutritional status of the whole animal.
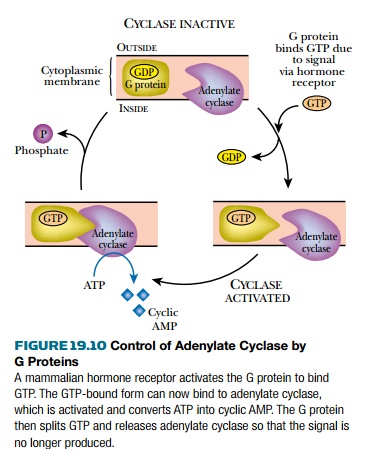
This is frequently mediated by the coupling of a hormone receptor to a G
protein n the cell membrane. G proteins were so named because their mechanism
depends on binding GTP when activated. They gothrough a cycle in which the
bound GTP is split to give GDP and phosphate ( Fig. 19.10 ). Thus, cyclic AMP
acts as a second messenger in response to signals delivered to the cell by a
hormone.
In both animals and bacteria, cyclic AMP
increases transcription of certain genes. In bacteria, cAMP binds to CRP
protein . This is a DNA binding protein that acts as a global gene activator. In
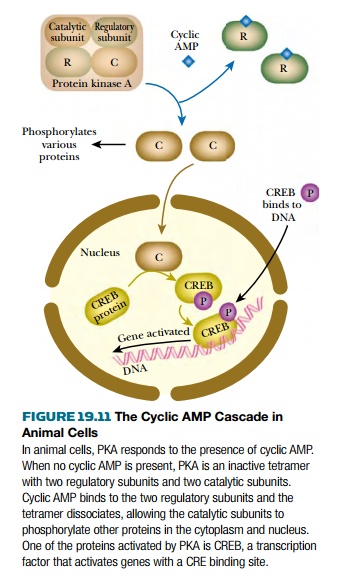
animal cells, cyclic AMP not only affects gene expression but also directly
affects enzyme activity ( Fig. 19.11 ). In animals, accumulation of cyclic AMP
activates protein kinase A (PKA) . In its inactive form this enzyme consists of
two regulatory (R) subunits and two catalytic (C) subunits. When cyclic AMP
binds to the R-subunits, the C-subunits are released and proceed to
phosphorylate a variety of target proteins, depending on the cell type. Some of
these are enzymes and are located in the cytoplasm. Phosphorylation may cause
activation or deactivation, depending on the particular enzyme. In addition, a few
active C-subunits enter the nucleus where they phosphorylate the CREB protein
(cyclic AMP response element binding protein) . The phosphorylated form of CREB
binds to a specific DNA sequence, the CRE (cyclic AMP response element) found
in front of those genes that are activated by cyclic AMP.
Related Topics