Chapter: Biotechnology Applying the Genetic Revolution: Noninfectious Diseases
Cloning and Genetic Engineering of Insulin
CLONING
AND GENETIC ENGINEERING OF INSULIN
Insulin was the first genetically
engineered hormone to be made commercially available for human use. Before
cloned human insulin was available, people with diabetes had to give themselves
injections of insulin extracted from the pancreas of animals such as cows or
pigs. Although this worked well on the whole, occasional allergic reactions
occurred, usually to low-level contaminants in the extracts. Today, genuine
human insulin (Humulin, marketed by Eli Lilly Inc.) made by recombinant bacteria
is available.
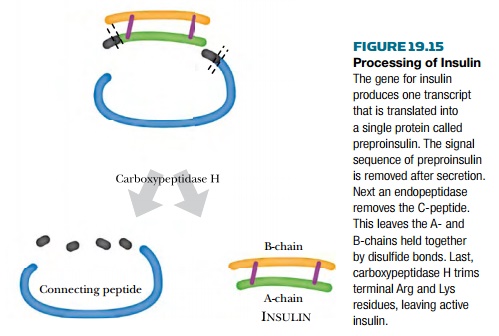
If the insulin gene is cloned and
directly expressed in bacteria, preproinsulin would be made. Because bacteria
lack the mammalian processing enzymes, the preproinsulin would not be converted
into insulin (see Fig. 19.15 ). In practice, there are two possible solutions
to this problem. The first is to purify the preproinsulin and then treat it with
enzymes that convert it into insulin. This means the processing enzymes must be
manufactured as well. Clearly this is overly complex. The solution chosen was
to make two artificial mini-genes , one for the insulin A-chain and the other for
the insulin B-chain ( Fig. 19.18 ).
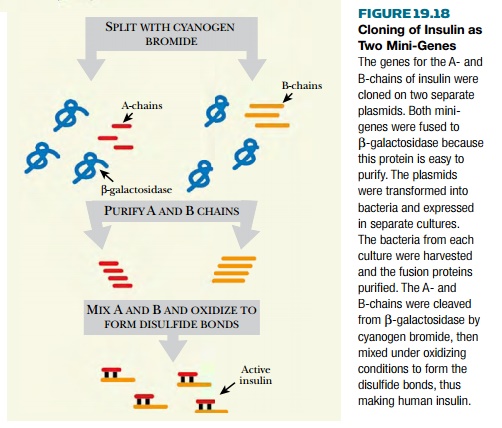
Two pieces of DNA, encoding the two insulin
chains, were synthesized chemically. The two DNA molecules were inserted into
plasmids that were put into two separate bacterial hosts. Thus, the two chains
of insulin were produced separately by two bacterial cultures. They were then
mixed and treated chemically to generate the disulfide bonds linking the chains
together.
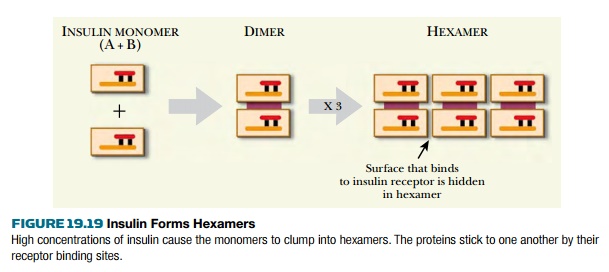
The approach just described gives
insulin that works well. Nonetheless, natural insulin, even natural human
insulin, is not perfect, and we can improve on nature. The problem is that
natural insulin tends to form hexamers. This clumping covers up the surfaces by
which the insulin molecule binds to the insulin receptor, thus preventing most of
the insulin from activating its target cells ( Fig. 19.19 ). In vivo, insulin
is secreted from the pancreas as a monomer and is distributed rapidly by the
bloodstream before it gets a chance to clump. However, when insulin is
injected, a high concentration of insulin is present in the syringe and
clumping occurs. After injection, it takes a while for the hexamers to
dissociate, and it may take several hours for the patient’s blood glucose to
drop to normal levels.
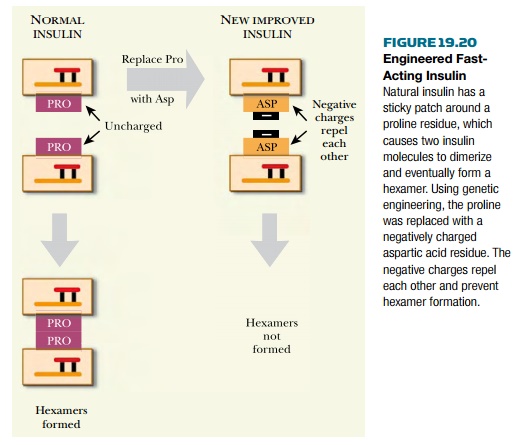
Insulin can be genetically engineered to
prevent clumping. The DNA sequence of the insulin gene is altered to change the
amino acid sequence of the resulting protein. A proline located at the surface
where the insulin molecules touch each other when forming the hexamer is
replaced with aspartic acid, whose side chain carries a negative charge. So when
twomodified insulin molecules approach each other, they are mutually repelled
by their negative charges and no longer clump ( Fig. 19.20 ). The altered
insulin causes a faster drop in blood sugar than native insulin. In 1999 the
Danish pharmaceutical company Novo received approval from the European Union,
and the improved insulin may eventually replace the natural product.
Related Topics