Chapter: Biotechnology Applying the Genetic Revolution: Bacterial Infections
Antitoxin Therapy
ANTITOXIN
THERAPY
Even if infections occur and toxins are
secreted by the invading bacteria, it may be possible to protect the patient against
the toxins. Traditional antitoxin treatment has relied on antibodies against
bacterial toxins. However, new geneoriented approaches are emerging.
One approach relies on dominant-negative
mutations in the binding subunit of the toxin. Defective mutations typically
result in proteins that are inactive. However, occasional mutations give rise
to proteins that not only are inactive themselves, but interfere with the
functional version of the protein. The presence of such a mutation in the same cell
as the wild-type version of the gene results in absence of activity—hence the
term dominant-negative .
The mechanism usually involves the binding
of a defective protein subunit to functional subunits resulting in a complex that
is inactive overall. Consequently, most dominantnegative mutations affect proteins
with multiple subunits. The multisubunit B proteins of A and B toxins such as
cholera toxin and anthrax toxin are good examples. Dominant-negative mutations
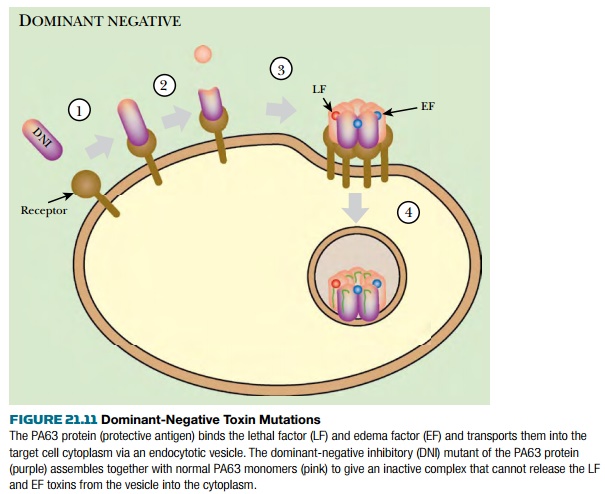
have been deliberately isolated in the B protein (i.e., the protective antigen,
PA) of anthrax toxin. Mixing mutant subunits with active ones resulted in the
assembly of inactive heptamers that bind the lethal or edema factor proteins
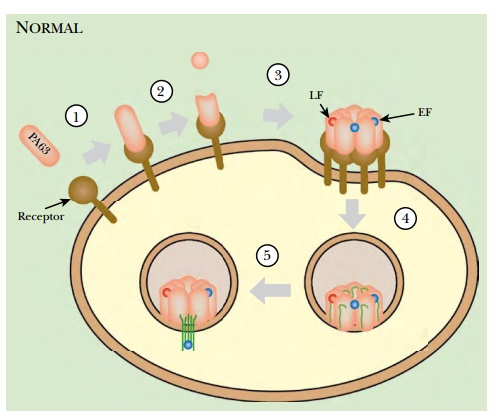
(i.e., the A subunits) but cannot transport them into target cells ( Fig. 21.11
). Treatment with the dominant-negative PA protein protected both cultured human
cells and whole mice or rats from death by lethal levels of anthrax toxin.
Another approach is the use of phage
display to isolate nonnatural peptides
that bind to bacterial toxins. Such peptides typically bind rather weakly to
single proteins.
However, if several copies of the peptide
are attached to a flexible backbone, this gives what is known as a polyvalent
inhibitor . Binding to multiple target proteins will occur, which results in
massively increased overall binding affinity. As discussed earlier, for this to
work, the target must be a multisubunit protein such as the heptameric PA
protein of anthrax toxin. Polyvalent peptide inhibitors with a polyacrylamide backbone
have proven successful in protecting animals against anthrax toxin.
Related Topics