Chapter: Biotechnology Applying the Genetic Revolution: Bacterial Infections
Attachment and Entry of Pathogenic Bacteria
ATTACHMENT
AND ENTRY OF PATHOGENIC BACTERIA
The first step in many infections is the
binding of bacteria to the surface of cells of the host animal. This is
mediated by proteins known as adhesins that usually bind to sugar residues of
glycoproteins or glycolipids on the animal cell surface. There are two major
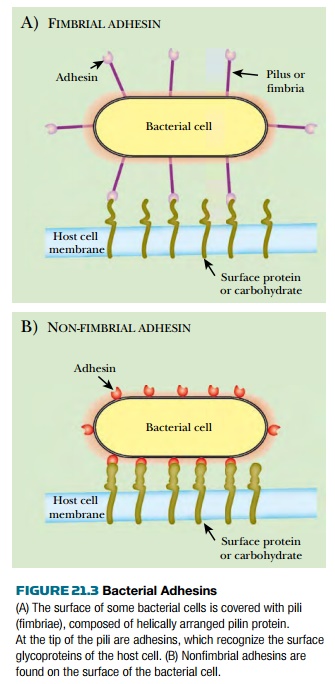
types of adhesins, fimbrial adhesins and nonfimbrial adhesins ( Fig. 21.3 ).
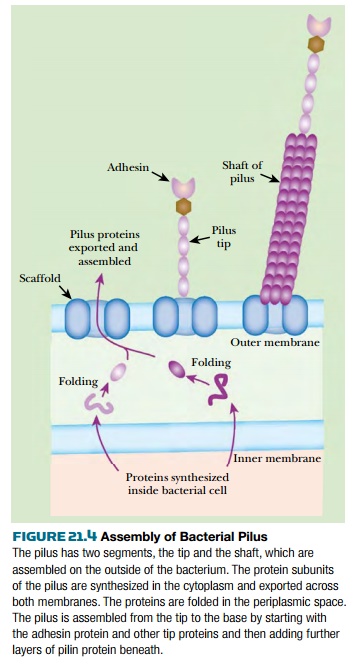
Pili (singular, pilus ) or fimbriae (singular, fimbria ) are thin
filaments that protrude from the surface of bacteria ( Fig. 21.4 ). The shaft
is composed of helically arranged subunits of protein ( pilin ). Several specialized
proteins, including adhesins , are carried at the very tip. Nonfimbrial
adhesins are found on the surface of bacterial cells. In many cases, pili make
first contact with the host cell and the nonfimbrial adhesins are responsible
for a later and closer stage of binding.
A second common step of infection is
entering an animal cell. Not all bacteria that adhere to animal cell surfaces
possess the ability to invade. Some pathogens remain permanently outside the
host cells. A classic example is cholera. Here the bacteria remain in the lumen
of the intestine attached to the outside of intestinal cells. Only the toxin
enters the intestinal cells and is responsible for the symptoms of the disease
(see later discussion). Other pathogens possess various strategies for entering
host cells. In some cases animal cells that are normally phagocytic, such as
many ameboid cells of the immune system, ingest the bacteria but fail to
destroy them. In other cases, bacteria provoke animal cells to swallow them by
means of proteins known as invasins . For example, the invasin of Yersinia binds
to the integrin proteins on mammalian cell surfaces and promotes internalization
of the bacteria.
The spread of antibiotic resistance has provoked scientists to consider alternative approaches to treating infections. Several proposals have been made that take advantage of adhesins and invasins to turn the tables on bacterial invaders. Because these are still mostly in the experimental phase, we will outline a few examples briefly.
Binding studies combined with x-ray
crystallography can reveal details of the molecular targets for adhesins. Thus,
the FimH adhesin of pathogenic Escherichia coli binds mannose residues
on the surface of mammalian glycoproteins. Several alkyl- and aryl-mannose derivatives
bind with extremely high affinity to the adhesin and can block attachment to
the natural receptor. Mannose derivatives might form the basis for designing
antiadhesin drugs that prevent bacterial binding.
A further suggested step is to
genetically engineer harmless gut bacteria, such as nonpathogenic strains of E.
coli , to express the target oligosaccharide for adhesins on their cell
surfaces. Pathogenic bacteria would then bind to these decoys instead of to
mammalian cells. This would avoid the need for administration of expensive
sugar derivatives, because the decoy strains of E. coli would multiply
naturally in the intestine. Furthermore, one engineered decoy strain could
carry multiple adhesin targets.
A third possibility is to equip
nonpathogenic strains of E. coli with genes for adhesins and/or invasins
from pathogens. These harmless strains would compete with the pathogens and
block the receptors. Such engineered strains could also be used to deliver
protein pharmaceuticals or large segments of DNA for gene therapy into
mammalian cells. Once inside, the engineered E. coli would be digested
by the mammalian cell and its therapeutic payload would be released.
Related Topics