Chapter: Medical Surgical Nursing: Management of Patients With Burn Injury
Acute or Intermediate Phase of Burn Care: Grafting the Burn Wound
Grafting the Burn Wound
If
wounds are deep (full-thickness) or extensive, spontaneous re-epithelialization
is not possible. Therefore, coverage of the burn wound is necessary until
coverage with a graft of the patient’s own skin (autograft) is possible. The purposes of wound coverage are to
decrease the risk for infection; prevent further loss of protein, fluid, and
electrolytes through the wound; and minimize heat loss through evaporation.
Several methods of wound coverage are available; some are temporary until grafting
with permanent cov-erage is possible. Wound coverage may consist of biologic,
bio-synthetic, synthetic, and autologous methods or a combination of these
approaches.
The
main areas for skin grafting include the face (for cosmetic and psychological
reasons); functional areas, such as the hands and feet; and areas that involve
joints. Grafting permits earlier functional ability and reduces contractures (shrinkage of burn scar
through collagen maturation). When burns are very exten-sive, the chest and
abdomen may be grafted first to reduce the burn surface.
Granulation
tissue fills the space created by the wound, cre-ates a barrier to bacteria,
and serves as a bed for epithelial cell growth. Richly vascular granulation
tissue is pink, firm, shiny, and free of exudate and debris. It should have a
bacterial count of less than 100,000 per gram of tissue to optimize graft take.
If the wound is not ready for skin grafting, the burn wound is excised and
allowed to granulate. Once the wound is excised, a wound covering is applied to
keep the wound bed moist and promote the granulation process.
BIOLOGIC DRESSINGS (HOMOGRAFTS AND HETEROGRAFTS)
Biologic dressings have several uses. In extensive
burns, they save lives by providing temporary wound closure and protecting the
granulation tissue until autografting is possible. Biologic dress-ings are
commonly used in patients with large areas of burn and little remaining normal
skin donor sites. Biologic dressings mayalso be used to débride
wounds after eschar separation. With each biologic dressing change, débridement
occurs. Once the biologic dressing appears to be “taking,” or adhering to the
granulating surface with minimal underlying exudation, the patient is ready for
an autograft.
Biologic dressings also provide temporary immediate
coverage for clean, superficial burns and decrease the wound’s evaporative
water and protein loss. They decrease pain by protecting nerve endings and are
an effective barrier against water loss and entry of bacteria. When applied to
superficial partial-thickness wounds, they seem to speed healing. Biologic
materials can be left open or covered. They stay in place for varying lengths
of time but are re-moved in instances of infection or rejection.
Biologic
dressings consist of homografts (or
allografts) and heterografts (or
xenografts). Homografts are skin obtained fromliving or recently deceased
humans. The amniotic membrane (amnion) from the human placenta may also be used
as a biologic dressing. Heterografts consist of skin taken from animals
(usually pigs). Most biologic dressings are used as temporary coverings of burn
wounds and are eventually rejected because of the body’s immune reaction to
them as foreign.
Homografts tend to be the most expensive biologic
dressings. They are available from skin banks in fresh and cryopreserved
(frozen) forms. Homografts are thought to provide the best in-fection control
of all the biologic or biosynthetic dressings avail-able. Revascularization
occurs within 48 hours, and the graft may be left in place for several weeks.
Cost, availability, and the pos-sibility of transmission of disease limit the
use of homografts.
Amnion
is less expensive and is available in hospitals with burn centers and
specialized tissue banks, which obtain and process it in cooperation with
obstetric services. However, amnion grafts do not become vascularized by the
patient’s vessels and can be left in place only for short periods.
Pigskin is available from commercial suppliers. It
is available fresh, frozen, or lyophilized (freeze-dried) for longer shelf
life. Pigskin impregnated with a topical antibacterial agent such as sil-ver
nitrate is also available. Pigskin is widely used for temporary covering of
clean wounds such as superficial partial-thickness wounds and donor sites.
Although pigskin does not vascularize, it will adhere to clean superficial
wounds and provides excellent pain control while the underlying wound
epithelializes.
BIOSYNTHETIC AND SYNTHETIC DRESSINGS
Problems with availability, sterility, and cost
have prompted the search for biosynthetic and synthetic skin substitutes, which
may eventually replace biologic dressings as temporary wound coverings.
Currently the most widely used synthetic dressing is Biobrane, which is composed of a nylon, Silastic membrane combined
with a collagen derivative. The material is semitransparent and sterile. It has
an indefinite shelf life and is less costly than homograft or pigskin. Like
biologic dressings, Biobrane protects the wound from fluid loss and bacterial
invasion.
Biobrane adheres to the wound fibrin, which binds to the nylon–collagen material. Within 5 days, cells migrate into the nylon mesh. Generally, adherence to the wound surface correlates directly with low bacterial counts. When the Biobrane dressing adheres to the wound, the wound remains stable and the Bio-brane can remain in place for 3 to 4 weeks. Biobrane dressings (Fig. 57-4) readily adhere to donor sites and meticulously clean débrided partial-thickness wounds; they will remain until spon-taneous epithelialization and wound healing occur. Biobrane can be laid on top of a wide-meshed autograft to protect the wound until the autograft epithelium grows out to close the interstices. As the Biobrane gradually separates, it is trimmed, leaving a healed wound.
Biobrane
is also useful for intermediate or long-term closure of a surgically excised
wound until an autograft becomes avail-able. Like biologic dressings, Biobrane
should not be used over grossly contaminated or necrotic wounds. Removal of
Biobrane after several weeks is similar to but easier than removal of a
vas-cularized allograft and leaves a bleeding granulation bed that readily
accepts an autograft.
Another fairly new temporary wound covering is BCG
Matrix. This dressing combines beta-glucan, a complex carbohydrate, with
collagen in a meshed reinforced wound dressing. Beta-glucan is known to
stimulate macrophages, which are vital in the in-flammatory process of healing.
BCG Matrix is a temporary wound covering intended for use with
partial-thickness burns and donor sites. It is applied immediately after
cleaning and débridement. If the burn wound surface remains free of infection,
BCG Matrix can be left in place until healing is complete.
Several
other synthetic dressings are available for burn wound care. Op-Site, a thin,
transparent, polyurethane elastic film, can be used to cover clean
partial-thickness wounds and donor sites. This dressing is occlusive and
waterproof but permeable to water vapor and air; this permeability not only
provides protection from microbial contamination but also allows for the
exchange of gases, which occurs much more quickly in a moist environment. Other synthetic dressings used for
burn wounds include Tegaderm, N-Terface, and DuoDerm. Burns that are between
superficial and deep partial thickness in depth can be treated with a promising
new temporary biologic covering, TransCyte, a material composed of human
newborn fi-broblasts, which are cultured on the nylon mesh of Biobrane. The
thin silicone membrane bonded to the mesh provides a moisture vapor barrier for
the wound. TransCyte is used to treat burns in which the depth is
indeterminate. TransCyte delivers a variety of biologically active proteins,
which may benefit the wound healing process. Research has shown that wounds
treated with TransCyte healed more quickly and with less hypertrophic scarring
than burns treated with the traditional silver sulfadiazine protocols
(Noordenbos, Dore & Hansbrough, 1999).
DERMAL SUBSTITUTES
In
an attempt to develop the ideal burn wound covering product, dermal substitutes
have been created. Two such products are In-tegra Artificial Skin and Alloderm.
Artificial skin (Integra) is the newest type of dermal substitute. A dermal analog,
Integra is composed of two main layers. The epi-dermal layer, consisting of
Silastic, acts as a bacterial barrier and prevents water loss from the dermis.
The dermal layer is composed of animal collagen. It interfaces with the open
wound surface and allows migration of fibroblasts and capillaries into the
material. This “neodermis” becomes a permanent structure. The artificial dermis
is biodegraded and reabsorbed. The outer silicone mem-brane is removed 2 to 3
weeks after application and is replaced with the patient’s own skin in the form
of a thin epidermal skin graft. Long-term effects of Integra include minimal
contracture formation. The graft site is very pliable, almost eliminating the
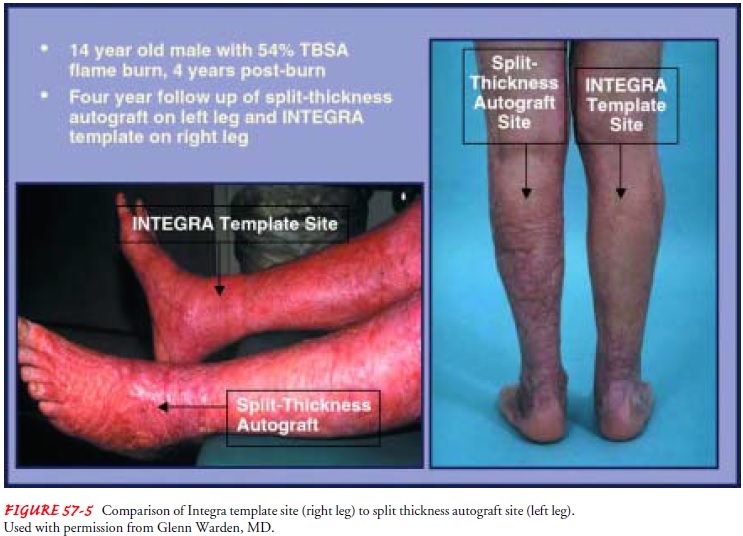
need for repeated cosmetic surgery. Most importantly, Integra has resulted in
less hypertrophic scarring (Fig. 57-5), thus eliminating the need for
compression devices once the burn wound has healed. The use of Integra is
increasing the survivability of burns and im-proving the functional and
cosmetic qualities of the healed burn (Winfrey, Cochran & Hegarty, 1999).
Another promising dermal substitute is Alloderm. It is processed dermis from
human cadaver skin, which can be used as the der-mal layer for skin grafts.
When a donor site (the area from which skin is taken to provide a skin graft
for another part of the body) is harvested for an autologous skin graft, both
the epidermal and dermal layers of skin are removed from the donor site.
Alloderm provides a permanent dermal layer replacement. Its use allows the burn
surgeon to harvest a thinner skin graft consisting of the epi-dermal layer
only. The patient’s epidermal layer is placed directly over the dermal base
(Alloderm). The new graft is then treated according to the burn unit’s
protocol. Use of Alloderm has also resulted in less scarring and contractures
with healed grafts; donor sites heal much more quickly than conventional donor
sites be-cause only the epidermal layer has been harvested. This is impor-tant
when donor sites are limited because of extensive burns (Luterman, 2000).
AUTOGRAFTS
Autografts remain the preferred material for
definitive burn wound closure following excision. Autografts are the ideal
means of cover-ing burn wounds because the grafts are the patient’s own skin
and thus are not rejected by the patient’s immune system. They can be
split-thickness, full-thickness, pedicle flaps, or epithelial grafts.
Full-thickness and pedicle flaps are commonly used for recon-structive surgery,
months or years after the initial injury.
Split-thickness autografts can be applied in sheets or in postage stamp–like pieces, or they can be expanded by meshing so that they can cover 1.5 to 9 times more than a given donor site area. Skin meshers enable the surgeon to cut tiny slits into a sheet of donor skin, making it possible to cover large areas with smaller amounts of donor skin.
These
expanded grafts adhere to the re-cipient site more easily than sheet grafts and
prevent the accumu-lation of blood, serum, air, or purulent material under the
graft. However, any kind of graft other than a sheet graft will contribute to
scar formation as it heals. Using expanded grafts may be neces-sary in large
wounds but should be viewed as a compromise in terms of cosmesis.
If
blood, serum, air, fat, or necrotic tissue lies between the re-cipient site and
the graft, there may be partial or total loss of the graft. Infection and
mishandling of the graft, as well as trauma during dressing changes, account
for most other instances of graft loss. Using split-thickness grafts allows the
remaining donor site to retain sweat glands and hair follicles and minimizes
donor site healing time.
Use
of cultured epithelial autograft
(CEA) is common at several burn centers. This involves a biopsy of the
patient’s skin in an unburned area. Keratinocytes are then isolated and
ep-ithelial cells are cultured in a laboratory. The original epithelial cell
sample can multiply to 10,000 times its original size over 30 days. These cells
are then attached to the burn wound. Vary-ing degrees of success have been
reported, and results are en-couraging. However, the disadvantages of the CEA
are that the grafts are thin and fragile and can shear easily. Research has
shown that the outcomes of use of CEA are not as positive as once thought. The
quality of burn scars is better, but patients have longer hospital stays and
higher hospital costs and require more surgical procedures than those treated
by traditional methods. In addition, patients require more reconstructive
procedures in the first 1 to 2 years postinjury. Therefore, CEA use is very
limited and reserved for burn patients whose donor sites are limited
(Noordenbos et al., 1999).
Care of the Patient with an Autograft.
Occlusive dressings are
commonly used initially after grafting to immobilize the graft. Occupational
therapists may be helpful in constructing splints to immobilize newly grafted
areas to prevent dislodging the graft. Homografts, heterografts, or synthetic
dressings may also be used to protect grafts. The graft may be left open with
skin staples to immobilize it, which allows close observation of progress.
The
first dressing change is usually performed 3 to 5 days after surgery, or
earlier in the case of purulent drainage or a foul odor. If the graft is
dislodged, sterile saline compresses will help prevent drying of the graft
until the physician reapplies it.
The patient is positioned and turned carefully to
avoid dis-turbing the graft or putting pressure on the graft site. If an
ex-tremity has been grafted, it is elevated to minimize edema. The patient
begins exercising the grafted area 5 to 7 days after grafting.
Care of Donor Site.
A moist gauze dressing is applied at the time of
surgery to maintain pressure and to stop any oozing. A throm-bostatic agent
such as thrombin or epinephrine may be applied directly to the site as well.
The donor site may be treated in sev-eral ways, from single-layer gauze
impregnated with petrolatum, scarlet red, or bismuth to new biosynthetic
dressings such as Bio-brane or BCG Matrix. Some burn centers are using the
Acticoat dressing on donor sites. Despite the type of donor site covering,
donor sites must remain clean, dry, and free from pressure. Be-cause a donor
site is usually a partial-thickness wound, it will heal spontaneously within 7
to 14 days with proper care. Donor sites are painful, and additional pain
management must be a part of the patient’s care.
Related Topics