Chapter: Genetics and Molecular Biology: Repression and the lac Operon
A Mechanism for Induction - Repression and the lac Operon
A Mechanism for Induction
How do inducers reduce repressor’s affinity for
operator? It is possible that they bind near the operator-binding site and
merely interfere with repressor’s correct binding to operator. This possibility
seems unlikely
in view of the genetic data and the separability of
the IPTG-binding and operator-binding substructures of repressor discussed
above. It seems more likely that IPTG merely causes the subunits of repressor
to alter positions slightly with respect to one another. Why should this
drasti-cally weaken the binding? Experiments with the N-terminal DNA-bind-ing
domain contain the answer.
The DNA-binding domain binds to operator with a
much lower affinity than wild-type repressor. Such reduced affinity actually is
expected. The wild-type tetrameric repressor molecule possesses a rela-tively
rigid structure in which pairs of the N-terminal regions are held in positions
appropriate for binding to a single operator. That is, the binding of one of
the N-terminal regions to half of the symmetrical operator perforce brings a
second N-terminal region into position for its binding to the other half of the
operator. Therefore most of the additional interaction energy between this
subunit and the DNA can be used to hold the complex onto the DNA (Fig. 11.12).
This is another
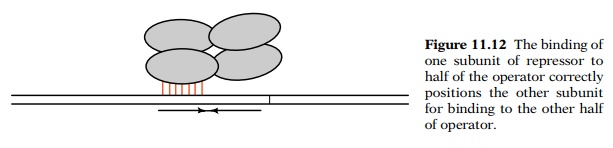
example of the chelate effect. Overall, the result
is that the oligomeric repressor tightly binds to operator. The same is not
true of the isolated N-terminal domains. The binding of one of these
DNA-binding domains does not automatically bring another into position for
binding to the other half of operator. As a result of their independent
binding, the apparent affinity of a single DNA-binding domain for operator is
low.
The chelate effect also streamlines the explanation
of induction: binding of IPTG shifts pairs of subunits away from optimal
relative positions for headpiece binding to operator. Consequently, the
affinity of repressor for operator is greatly reduced, and repressor
dissociates. Eventually, direct experiments may be able to test such ideas. In
the meantime, two types of repressor mutations are consistent with this point
of view. Repressor mutants can be found that are not located in the N-terminal
region and that result in much tighter or much weaker repressor-operator
binding. These mutants possess no discernible struc-tural alterations. Most
likely these types of mutation merely shift the positions of the subunits
slightly with respect to one another. The tighter-binding mutant must bring the
subunits into closer complemen-tarity with operator, and the weaker-binding
mutants must shift the subunits away from complementarity.
An enormous amount has been learned about the lac operon, and only a fraction was
mentioned. More remains to be learned about the physiology and physical
chemistry underlying regu-lation of this set of genes and others.
Related Topics