Chapter: Genetics and Molecular Biology: Repression and the lac Operon
An Assay for lac Repressor
An Assay for lac
Repressor
Genetic and physiological experiments investigating properties of the lac operon provided information from which a number of regulatory mechanisms were proposed. These ranged from the logical mechanism of lac repressor binding to DNA and inhibiting transcription to compli-cated translational control mechanisms utilizing tRNA molecules. Clear demonstration of the regulation mechanism required purification of its components and in vitro reconstruction of the lac system.
The most important step in the reconstruction of
the lac regulatory system was the
ability to detect repressor. Lac repressor, of course, had to be highly
purified from lysed cells. If regulation of the lac operon were efficient–and that is the main reason for the
existence of regulation–then the cell should contain far fewer molecules of
repressor than of the induced gene products. Furthermore, since lac repressor possessed no known
enzymatic activity, no easy and sensitive assay for repressor was available.
Without the ability to detect repressor, its purification was impossible
because any fraction obtained from purification steps that was enriched in
repressor could not be identified.
Repressor’s only known property was that it bound
inducer, IPTG being one. Therefore Gilbert and Müller-Hill developed an assay
of lac repressor based on the
protein’s ability to bind to inducer molecules. Equilibrium dialysis can detect
a protein that binds a particular small molecule. The protein solution to be
assayed is placed in a dialysis sack and dialyzed against a buffer that
contains salts to maintain the pH and ionic strength and the small molecule
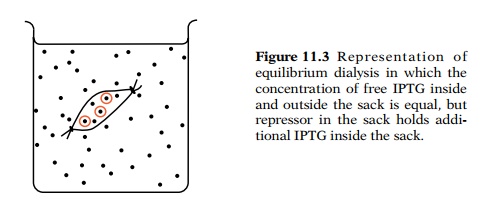
that binds to the protein (Fig. 11.3).
In the case of repressor, radioactive IPTG was
used. After equilibrium has been attained, the concentration of free IPTG
inside and outside the sack is equal, but in addition, inside the sack are the
molecules of IPTG that are bound to repressor. If the concentration of
repressor is suffi-ciently high, the increased amount of IPTG inside the sack
due to the presence of repressor can be detected. Both the inside and outside
concentrations of IPTG can be determined by measuring the amount of
radioactivity contained in samples of known volumes taken from out-side and
inside the dialysis sack.
Does an equilibrium dialysis assay possess
sufficient sensitivity to detect the small amounts of lac repressor that are likely to exist in crude
extracts of cells? The binding reaction between
repressor and IPTG is closely approximated by the reaction
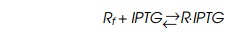
where Rf is the concentration of free repressor, IPTG is the concentra-tion of free IPTG,
and R⋅IPTG is the concentration of the complex between
repressor and IPTG. A dissociation constant KD describes the relations between the
concentrations:
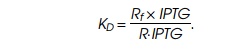
Substituting
the conservation equation, Rf+R⋅IPTG=Rt, where Rt is the total amount of
repressor, and rearranging yields the relation we need. Biochemists have many
different names for the equivalent algebraic rearrangements of this equation
but usually call the phenomenon Michaelis-Menten binding,
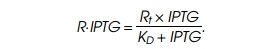
The ratio of radioactivity in the samples obtained from inside and
outside the sack is
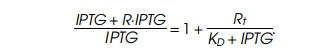
Normally in liquid scintillation, counting a 5%
difference between samples with more than 100 cpm can be readily determined.
Thus the quantity
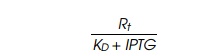
must be greater than 0.05 for detection of lac repressor by this assay.
Related Topics