Chapter: Genetics and Molecular Biology: Repression and the lac Operon
Detection and Purification of lac Repressor
Detection and Purification of lac Repressor
The previous section showed that
wild-type lac repressor in crude
extracts of cells was not likely to produce a detectable signal in the
equilibrium dialysis assay. Therefore Gilbert and Müller-Hill isolated a mutant
repressor that bound IPTG more tightly than the wild-type repressor. Crude
extracts made from this strain showed an excess of counts in the dialysis sack.
The excess was barely detectable; nonethe - less it was statistically
significant, and fractionation of the extract yielded a protein sample with an
easily detectable excess of counts.
Once the
assay of lac repressor detected
something, it was of great importance to prove that the origin of the signal
was repressor and not something else. The proof used the tight-binding mutant.
First, the tight-binding mutant was used to develop a partial purification of
repressor so that a fraction could be obtained in which the signal was readily
detectable. Then this same purification procedure was used to obtain a similar
fraction from wild-type cells. This too generated a significant signal. The
proof came with the demonstration that the apparent dissociation constants for
IPTG in the fraction from the mutant and the wild-type were different. This was
simply done by performing the dialysis on a series of samples at different
concentra-tions of IPTG. The sample obtained from the mutant bound IPTG more
tightly. That is, it had a smaller KD,
than the wild-type (Fig. 11.5). As the only difference between the mutant and
the wild-type was a mutation in the lacI
gene, the signal in the assay was from lac
repressor.
The
definitive detection of repressor opened the door to biochemical studies.
First, with an assay, the repressor could in principle be purified and used in
biochemical studies probing its mechanism of action. Second, it was possible to
attempt to isolate mutants that synthesized elevated quantities of repressor so
as to ease the burden of purification. With an assay, such candidates could be
identified.
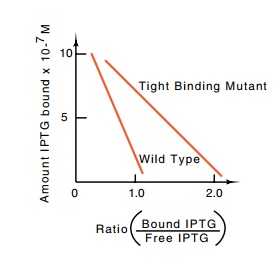
Figure
11.5 Results of equilib-rium dialysis
at different IPTG concentrations of wild-type re-pressor and a tight-binding
mutant repressor. Rearranging the binding equation derived in the text yields a
form convenient for plotting data, RI = R
- [K ×(RI)/I]. In this form thevalue of
RI when (RI)/I = 0 yields the concentration of R molecules capable of binding I
and the slope of the binding curve gives K.
A mutation rendering lac repressor temperature-sensitive was used in the isolation of
mutants possessing higher levels of repressor. Cells were grown at a
temperature just high enough to inactivate most of the temperature-sensitive
repressor. Consequently the lac
operon was no longer repressed, and the cells expressing the lac operon were then killed. The
survivors, which were able to repress the operon, could be of two types. Either
the repressor was altered so that it could repress at
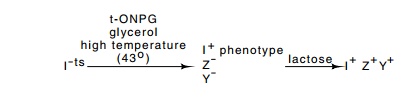
the
elevated temperature or more repressor was being synthesized. The two types of
mutants could easily be distinguished with the equilibrium dialysis assay, and
an overproducing mutant was identified.
The
selection for the loss of constitutivity in the scheme described above used yet
another lactose analog, TONPG (o-nitrophenyl-1-thio-β-D-galactoside). This inhibits
growth when it is cleaved by β-galactosi-dase,
but it is not an inducer. Mutant cells unable to cleave this compound grow in
its presence. Three types of mutants have this property: the desired repressing
mutants as well as lacZ and lacY mutants. Both of the undesired
mutant types were easily eliminated by requiring the mutants to grow on
lactose. The selection scheme was successful, and mutants were found that
contained elevated amounts of lac repressor.
The isolation of the lacI overproducer
was the first clearexample of the successful isolation of a promoter mutation
and was itself a breakthrough. The resulting IQ (Q for quantity)
mutation mapped at the beginning of the I gene, as expected for a promoter
mutation, and generated a 10-fold increase in the level of repressor.
Related Topics