Chapter: Genetics and Molecular Biology: Repression and the lac Operon
Difficulty of Detecting Wild Type lac Repressor
The Difficulty of Detecting Wild-Type lac Repressor
Before trying to detect lac repressor, Gilbert and Müller-Hill estimated the signal that
could be expected in the equilibrium assay and decided that they were unlikely
to detect wild-type repressor. Let us examine such a calculation. Two
quantities are needed: the dissociation constant of repressor for IPTG and the
concentration of repressor in cell extracts.
To make a crude guess of the dissociation constant
of IPTG from lac repressor, assume
that the basal level of the lac
operon is proportional to the fraction of lac
operator uncomplexed with repressor. Assume also that this fraction is doubled
if the effective repressor concentration is halved. Consequently, the basal
level of the operon is doubled when half the total repressor in the cell is
bound to IPTG and half is free of IPTG. The concentration of IPTG at which the
50-50 binding occurs and the enzyme level is twice the basal level equals the
dissociation constant for
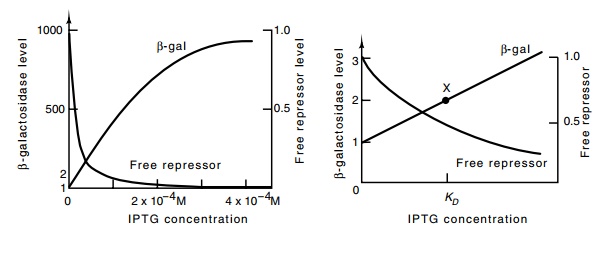
Figure
11.4 The relation between free
repressor concentration and the induc-tion level of the lac operon. X marks the position where [IPTG] = KD. At this point repressor concentration has fallen to half, and the
basal level has doubled.
IPTG
binding to repressor. Although this concentration of IPTG could be measured in
reasonably straightforward experiments, it can be calculated from data already
known. The lac operon can be induced
a thousandfold and is half-maximally induced by 2 × 10-4 M IPTG (Fig.
11.4). Roughly, then, at an IPTG concentration of (2 × 10-4)/500 = 4 × 10-7 M IPTG, the
level of expression of the lac operon
will be twice the basal level. Consequently an estimate of the dissociation
constant of lac repressor and IPTG is
4 × 10-7
M.
The
volume of a cell is 10-12 cm3 = 10-15 liter.
Using this value, a packed cell pellet is 1015/(6 × 1023), or about 10-9
M in cells. If a cell contains 10 repressor molecules, the concentration of
repressor in a packed cell pellet is 10-8 M. A cell lysate cannot
easily be made at a higher concen-tration than that obtained by opening cells
in a packed cell pellet. Hence a reasonable estimate for the concentration of
repressor in the equilib-rium dialysis assay is 10-8 M.
If high specific activity radioactive IPTG is available, it can be used in the assay at a concentration well below the KD estimated above . Therefore the ratio of the radioactivities contained in samples of equal volumes taken from inside and outside the sack is

which is less than can be reliably detected.
Related Topics