Chapter: Biotechnology Applying the Genetic Revolution: Proteomics
Protein Interactions by Co-immunoprecipitation
PROTEIN
INTERACTIONS BY CO-IMMUNOPRECIPITATION
Co-immunoprecipitation is a technique to examine protein interactions in the cytoplasm rather than the nucleus (Fig. 9.24). Here, the target protein is expressed in cultured mammalian cells, which are lysed to release the cytoplasmic contents. The target protein is precipitated from the lysate with an antibody. Other proteins that are associated with the target protein remain associated with the antibody-protein complex. (If no antibody exists for the target protein, a small tag [such as FLAG or His6; see earlier discussion] can be engineered onto the protein.) Protein A from Staphylococcus, in turn, binds the antibodies. The protein A is attached to beads before it is added to the cell lysate. This generates very large target protein/antibody/ protein A/bead complexes, which are gently isolated from the rest of the cellular proteins by centrifugation. The complexes are separated by size with SDS-PAGE. The gel should show the target protein, the antibody, protein A, and other bands that represent interacting proteins. These can be identified with protein sequencing and/or mass spectrometry.
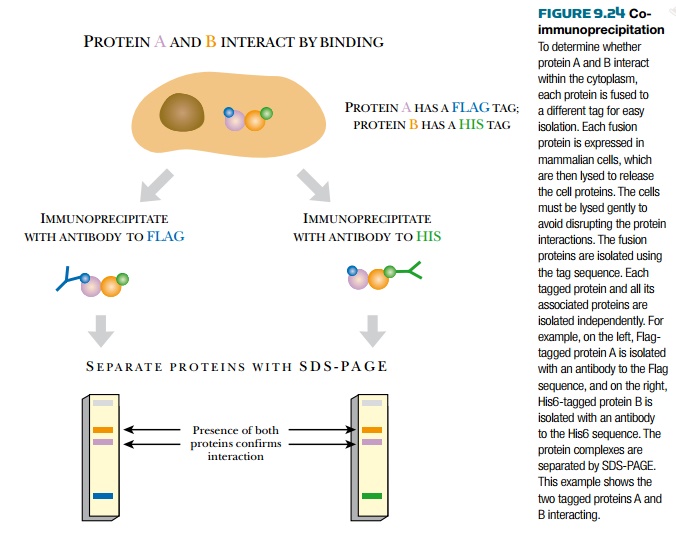
Co-immunoprecipitation is
often used to confirm the results from yeast two-hybrid analysis, especially
for mammalian proteins. Many two-hybrid experiments reveal novel
uncharacterized proteins. To confirm the interaction, both proteins are tagged
for easy isolation. Adding a tag is much easier than generating a specific
antibody to each new protein. For example, protein A is tagged with FLAG, while
protein B is tagged with His6. (Note: This protein A is not the protein A from
Staphylococcus.) Each vector construct is transformed into a mammalian cell
line, and each protein is expressed. The cell lysate is harvested and divided
into two samples. The protein A complexes are isolated from the first sample,
whereas the protein B complexes are isolated from the second sample. Each of
the complexes is isolated with protein A–coated beads. The different proteins
from each sample are separated by SDS- PAGE. If the two proteins interact, both
proteins will be found in both samples.
Related Topics