Chapter: Basic & Clinical Pharmacology : Hypothalamic & Pituitary Hormones
Gonadotropins & Human Chorionic Gonadotropin
THE GONADOTROPINS
(FOLLICLE-STIMULATING HORMONE & LUTEINIZING HORMONE) & HUMAN CHORIONIC
GONADOTROPIN
The gonadotropins are
produced by a single type of pituitary cell, the gonadotroph. These hormones
serve complementary func-tions in the reproductive process. In women, the
principal func-tion of FSH is to direct ovarian follicle development. Both FSH
and LH are needed for ovarian steroidogenesis. In the ovary, LH stimulates
androgen production by theca cells in the follicular stage of the menstrual
cycle, whereas FSH stimulates the conver-sion by granulosa cells of androgens
to estrogens. In the luteal phase of the menstrual cycle, estrogen and
progesterone produc-tion is primarily under the control first of LH and then,
if preg-nancy occurs, under the control of human chorionic gonadotropin (hCG).
Human chorionic gonadotropin is a placental protein nearly identical with LH;
its actions are mediated through LH receptors.
In
men, FSH is the primary regulator of spermatogenesis, whereas LH is the main
stimulus for testosterone synthesis in Leydig cells. FSH helps maintain high
local androgen concentrations in the vicinity of developing sperm by
stimulating the production of androgen-binding protein in Sertoli cells. FSH
also stimulates the conversion by Sertoli cells of testosterone to estrogen.
FSH,
LH, and hCG are available in several pharmaceutical forms. They are used in
states of infertility to stimulate spermato-genesis in men and to induce
ovulation in women. Their most common clinical use is for the controlled
ovulation hyperstimula-tion that is the cornerstone of assisted reproductive
technologies such as in vitro fertilization (IVF).
Chemistry & Pharmacokinetics
All three
hormones—FSH, LH, and hCG—are heterodimers that share an identical α chain in addition to
a distinct β
chain that confers receptor specificity. The β chains of hCG and LH are nearly identical,
and these two hormones are used interchange-ably. All the gonadotropin
preparations are administered by sub-cutaneous or intramuscular injection,
usually on a daily basis. Half-lives vary by preparation and route of injection
from 10 to 40 hours.
A. Menotropins
The first commercial
gonadotropin product was extracted from the urine of postmenopausal women,
which contains a substance with FSH-like properties (but with 4% of the potency
of FSH) and an LH-like substance. This purified extract of FSH and LH is known
as menotropins, or human menopausal
gonadotropins (hMG).
B. Follicle-Stimulating Hormone
Three forms of
purified FSH are available. Urofollitropin,
also known as uFSH, is a purified preparation of human FSH extracted from the
urine of postmenopausal women. Virtually all the LH activity has been removed
through a form of immuno-affinity chromatography that uses anti-hCG antibodies.
Two recombinant forms of FSH (rFSH)
are also available: follitropinalfa and follitropin beta. The amino acid
sequences of these twoproducts are identical to that of human FSH. They differ
from each other and urofollitropin in the composition of carbohydrate side
chains. The rFSH preparations have a shorter half-life than preparations
derived from human urine but stimulate estrogen secretion at least as
efficiently and, in some studies, more effi-ciently. The rFSH preparations are
considerably more expensive.
C. Luteinizing Hormone
Lutropin alfa, the recombinant form of human LH, was intro-duced in the United
States in 2004. When given by subcutaneous injection, it has a half-life of
about 10 hours. Lutropin has only been approved for use in combination with follitropin
alfa for stimulation of follicular development in infertile women with profound
LH deficiency. It has not been approved for use with the other preparations of
FSH nor for simulating the endogenous LH surge that is needed to complete
follicular development and pre-cipitate ovulation.
D. Human Chorionic Gonadotropin
hCG is produced by the
human placenta and excreted into the urine, whence it can be extracted and
purified. It is a glycoprotein consisting of a 92-amino-acid α chain virtually
identical to that of FSH, LH, and TSH, and a β chain of 145 amino acids that resembles that
of LH except for the presence of a carboxyl terminal sequence of 30 amino acids
not present in LH. Choriogonadotropin
alfa (rhCG) is a recombinant form ofhCG. Because of its greater consistency
in biologic activity, rhCG is packaged and dosed on the basis of weight rather
than units of activity. All of the other gonadotropins, including rFSH, are
pack-aged and dosed on the basis of units of activity. The preparation of hCG
that is purified from human urine is administered by intramuscular injection,
whereas rhCG is administered by subcu-taneous injection.
Pharmacodynamics
The gonadotropins and
hCG exert their effects through G protein-coupled receptors. LH and FSH have
complex effects on reproductive tissues in both sexes. In women, these effects
change over the time course of a menstrual cycle as a result of a complex
interplay between concentration-dependent effects of the gonadotropins,
cross-talk between LH, FSH, and gonadal steroids, and the influence of other
ovarian hormones. A coordinated pattern of FSH and LH secretion during the
menstrual cycle (see Figure 40–1) is required for normal follicle development,
ovula-tion, and pregnancy.
During the first 8
weeks of pregnancy, the progesterone and estrogen required to maintain
pregnancy are produced by the ovarian corpus luteum. For the first few days
after ovulation, the corpus luteum is maintained by maternal LH. However, as
mater-nal LH concentrations fall owing to increasing concentrations of
progesterone and estrogen, the corpus luteum will continue tofunction only if
the role of maternal LH is taken over by hCG produced by the embryo and its new
placenta.
Clinical Pharmacology
A. Ovulation Induction
The gonadotropins are
used to induce ovulation in women with anovulation that is secondary to
hypogonadotropic hypogo-nadism, polycystic ovary syndrome, obesity, and other
causes. Because of the high cost of gonadotropins and the need for close monitoring
during their administration, they are generally reserved for anovulatory women
who fail to respond to other less com-plicated forms of treatment (eg,
clomiphene;). Gonadotropins are also used for controlled ovarian hyperstimu-lation in assisted reproductive
technology procedures. A numberof protocols make use of gonadotropins in
ovulation induction and controlled ovulation hyperstimulation, and new
protocols are continually being developed to improve the rates of success and
to decrease the two primary risks of ovulation induction: multiple pregnancies
and the ovarian hyperstimulation
syndrome (OHSS; ).
Although the details
differ, all of these protocols are based on the complex physiology that
underlies a normal menstrual cycle. Like a menstrual cycle, ovulation induction
is discussed in rela-tion to a cycle that begins on the first day of a
menstrual bleed (Figure 37–3). Shortly after the first day (usually on day 3),
daily injections with one of the FSH preparations (hMG, urofollitro-pin) are
begun and are continued for approximately 7–12 days. In women with
hypogonadotropic hypogonadism, follicle develop-ment requires treatment with a
combination of FSH and LH because these women do not produce the basal level of
LH that is required for adequate ovarian estrogen production and normal
follicle development. The dose and duration of FSH treatment are based on the
response as measured by the serum estradiol concen-tration and by ultrasound
evaluation of ovarian follicle develop-ment and endometrial thickness. When
exogenous gonadotropins are used to stimulate follicle development, there is
risk of a pre-mature endogenous surge in LH owing to the rapidly changing
hormonal milieu. To prevent this, gonadotropins are almost always administered
in conjunction with a drug that blocks the effects of endogenous GnRH—either
continuous administration of a GnRH agonist, which down-regulates GnRH
receptors or a GnRH receptor antagonist (see below and Figure 37–3).
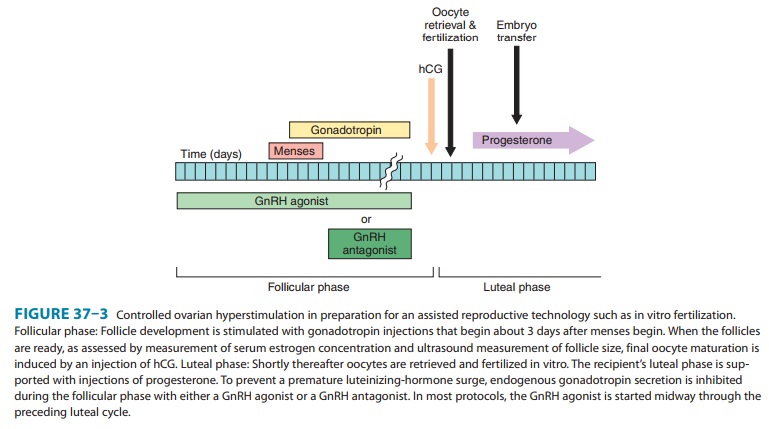
When appropriate follicular maturation has occurred, the FSH and the GnRH agonist or GnRH antagonist injections are discon-tinued; the following day, hCG (5000–10,000 IU) is administered intramuscularly to induce final follicular maturation and, in ovu-lation induction protocols, ovulation. The hCG administration is followed by insemination in ovulation induction and by oocyte retrieval in assisted reproductive technology procedures. Because use of GnRH agonists or antagonists during the follicular phase of ovulation induction suppresses endogenous LH production, it is important to provide exogenous hormonal support of the luteal phase. In clinical trials, exogenous progesterone, hCG, or a combination of the two have been effective at providing adequate luteal support. However, progesterone is preferred for luteal.
B. Male Infertility
Most of the signs and
symptoms of hypogonadism in males (eg, delayed puberty, retention of
prepubertal secondary sex char-acteristics after puberty) can be adequately
treated with exogenous androgen; however, treatment of infertility in
hypogonadal men requires the activity of both LH and FSH. For many years,
con-ventional therapy has consisted of initial treatment for 8–12 weeks with
injections of 1000–2500 IU hCG several times per week. After the initial phase,
hMG is injected at a dose of 75–150 units three times per week. In men with
hypogonadal hypogonadism, it takes an average of 4–6 months of such treatment
for sperm to appear in the ejaculate. With the more recent availability of
uro-follitropin, rFSH, and rLH, a number of alternative protocols have been
developed. An advance that has indirectly benefited gonadotropin treatment of
male infertility is intracytoplasmic sperm injection (ICSI), in which a single
sperm is injected directly into a mature oocyte that has been retrieved after
controlled ovar-ian hyperstimulation of a female partner. With the advent of
ICSI, the minimum threshold of spermatogenesis required for preg-nancy is
greatly lowered.
C. Outdated Uses
Chorionic gonadotropin
is approved for the treatment of prepuber-tal cryptorchidism. Prepubertal boys
generally between 4 and 9 years of age were treated with IM injections of hCG
for 2–6 weeks.
However, this clinical
use is no longer supported because the long-term efficacy of hormonal treatment
of cryptorchidism (∼ 20%) is much lower
than the long-term efficacy of surgical treatment (> 95%), and because of
concerns that early childhood treatment with hCG treatment has a negative
impact on germ cells in addition to increasing the risk of precocious puberty.
In the United States,
chorionic gonadotropin has a black-box warning against its use for weight loss.
The use of hCG plus severe calorie restriction for weight loss was popularized
by a publication in the 1950s claiming that the hCG selectively mobilizes body
fat stores. This practice continues today, despite a preponderance of
subsequent scientific evidence from placebo-controlled trials that hCG does not
provide any weight loss benefit beyond the weight loss associated with severe
calorie restriction alone.
Toxicity & Contraindications
In women treated with
gonadotropins and hCG, the two most seri-ous complications are the ovarian hyperstimulation syndrome and multiple pregnancies. Overstimulation
of the ovary during ovulation induction often leads to uncomplicated ovarian
enlarge-ment that usually resolves spontaneously. The ovarian hyperstimu-lation
syndrome is a more serious complication that occurs in 0.5–4% of patients. It
is characterized by ovarian enlargement, ascites, hydrothorax, and hypovolemia,
sometimes resulting in shock. Hemoperitoneum (from a ruptured ovarian cyst),
fever, and arterial thromboembolism can occur.
The probability of
multiple pregnancies is greatly increased when ovulation induction and assisted
reproductive technologiesare used. In ovulation induction, the risk of a
multiple pregnancy is estimated to be 15–20%, whereas the percentage of multiple
pregnancies in the general population is closer to 1%. Multiple pregnancies
carry an increased risk of complications, such as gesta-tional diabetes,
preeclampsia, and preterm labor. For in vitro fertil-ization procedures, the
risk of a multiple pregnancy is primarily determined by the number of embryos
transferred to the recipient. A strong trend in recent years has been to
transfer fewer embryos.
Other reported adverse
effects of gonadotropin treatment are headache, depression, edema, precocious
puberty, and (rarely) production of antibodies to hCG. In men treated with
gonadotro-pins, the risk of gynecomastia is directly correlated with the level
of testosterone produced in response to treatment. An association between
ovarian cancer, infertility, and fertility drugs has been reported. However, it
is not known which, if any, fertility drugs are causally related to cancer.
Related Topics