Chapter: Biochemistry: Living Cell
Acids and bases
Acids and bases
According to the modern concept, an acid is
defined as that species which can donate H+ ions (protons) in solution and a
base is that species which can accept H+ ions. Since such transfer of protons
is reversible any acid which gives up its proton becomes a base, while any base
which accepts a proton becomes an acid. This theory was postulated by Bronsted
and Lowry in 1923. The following general equation can be written as
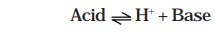
An acid and a base related in this manner are
called conjugates
For (eg) in this reaction
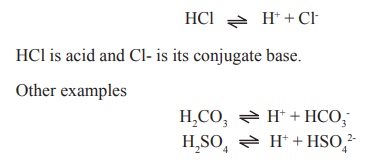
An acid which dissociates strongly and readily
gives H+ ions is known as a strong acid. The capacity of an acid to release its
protons is known as acidity.
A base which has more affinity to combine with
H+ ions is known as a strong base.
This property of a base is known as alkalinity
(eg) HCO3-, HSO4-, H2PO4-
etc.
The acidity of a species is denoted by its pH
value : larger the acidity of a species, lower will be its pH value.
pH - It is defined as the negative
logarithm of hydrogen ion concentration
(or) it is defined as the logarithm
of reciprocal of the hydrogen ion concentration.
i.e. pH = - log [H+] (or) pH = log
1/[H+]
Acidic - 1 - 6.9 Neutral 7.0 Alkaline 7.1 - 14.
Determination of pH -Henderson - Hasselbalch equation Derivation
This concerns the dissociation of weak acid in
equilibrium.
Let us consider HA a weak acid that ionises as
follows:
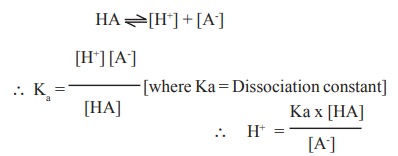
log [H+] = log Ka + log[HA]-log[A-]
(Taking log on both sides).
-log [H+] = -log Ka -log [HA] + [A-]
[Chaning sign on both sides].
pH = pKa + log [A-] / [HA] since -log H+ = pH and - log Ka =
pKa
The above equation is known as Henderson -
Hasselbalch equation. and can be used for the determination of pH of blood.
Determination of pH of buffers
The pH of buffers can be determined by
Henderson - Hasselbalch equation.
pH = pKa + log [salt] / [Acid]
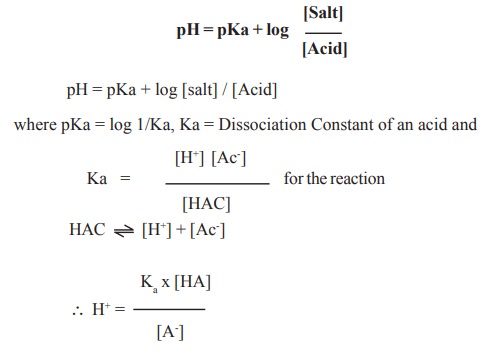
In case of blood, the ratio between [BHCO3]
: [H2CO3] can be found out by applying the above equation
to maintain average pH of blood 7.4. If the pKa value of H2CO3
is 6.1. then
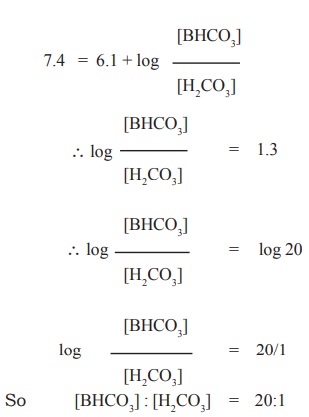
pH in living organisms
The fluid in the cells and tissues of plants
and animals is usually close to neutrality and the pH of extracellular fluids
under normal conditions varies from 7.35 to 7.5.
Regulations of acid-base balance (pH maintenance)
The following mechanisms control the regulation
of acid-base balance in the human body.
1. Buffer
system : Hemoglobin, phosphate and
carbonate - bicarbonate buffers are involved
in the maintenance of pH since they are capable of neutralising H+ or OH- ions
formed during metabolic activities.
2. Respiratory regulation : Lungs
are actually the
most effective organs
for pH
adjustment : One half of the H+ ions drained by
the cells to the extra cellular fluids combine with HCO3 - to form H2CO3
which dissociates in to H2O and CO2. The CO2
thus formed is expirated through lungs. So accumulation of H+ ions is
prevented.
3. Renal regulation
Kidney contribute for acid-base balance by
excreting acids and ammonia. The excess of acids produced in the metabolic
pathways are eliminated by kidneys in the form of urine. Ammonia is a base and
formed in the tubular epithelial cells from glutamine. Glutamine is deaminated
and then dehydrogenated to form two moles of ammonia. The ammonia so formed
diffuses into the tubular urine and binds H+ ions to form NH4+
and excreted as such in urine. If not excreted, ammonia accumulation in blood
leads to acid-base imbalance.
If acids and ammonia accumulate in the blood,
it lead to acidosis and alkalosis respectively.
Related Topics