Chapter: Biochemistry: Living Cell
Buffers of blood
Buffers of blood
The important buffers present in blood are
· Bicarbonate buffer
· Phosphate buffer
· Protein buffer
· Hemoglobin buffer
Bicarbonate buffer
It is the most important buffer in blood plasma and consist of bicarbonate [HCO3-] and carbonic acid [H2CO3] This buffer is efficient in maintaining the pH of blood plasma to 7.4 against the acids produced in tissue metabolism (eg) phosphoric acid, lactic acid, aceto acetic acid and b-hydroxy butyric acid. These acids are converted to their anions and the bicarbonate is converted to carbonic acid a weak acid.
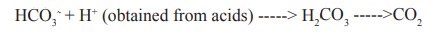
CO2 thus formed is expirated through lungs during respiration.
Phosphate buffer
The phosphate buffer consists of dibasic phosphate [HPO42-] and monobasic phosphate (H2PO4-). Its pKa value is about 6.8. It is more effective in the pH range 5.8 to 7.8. Plasma has a ratio of 4 between [HPO42-] : [H2PO4-].
Therefore pH = pKa + log { [HPO42-] /[H2PO4-] }
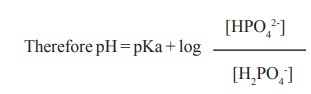
pH = 6.8 + log 4 = 7.4 [7.4 is the normal pH of blood]
Protein buffer
The protein buffers are very important in the plasma and in the intracellular fluids but their concentration is very low in CSF, lymph and interstitial fluids.
They exist as anions serving as conjugate bases (Pr-) at the blood pH 7.4 and form conjugate acids (HPr) accepting H+. They have the capacity to buffer some H2CO3 in the blood.
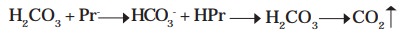
H2CO3 + Pr- -- > HCO3- + HPr - - > H2CO3 - - > CO2
Hemoglobin buffer
They are involved in buffering CO2 inside erythrocytes. The buffering capacity of hemoglobin depends on its oxygenation and deoxygenation. Inside the erythrocytes, CO2 combines with H2O to form H2CO3 under the action of carbonic anhydrase. At the blood pH 7.4, H2CO3 dissociates into H+ and HCO3- and needs immediate buffering. Oxyhemoglobin (HbO2-) on the other side loses O2 to form deoxyhemoglobin (Hb-) which remains undissociated (HHb) by accepting H+ from the ionization of H2CO3. Thus, Hb- buffers H2CO3 in erythrocytes.
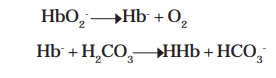
Related Topics