Chapter: Medical Electronics : Bio-Chemical and Non Electrical Parameter Measurement
pO2 Measurement
pO2 MEASUREMENT
The term pO2 is defined as the partial pressure of oxygen respectively. The
determination of pO2 is one the most important physiological chemical
measurement. The effective functioning of both respiratory and cardiovascular
system can be by pO2 measurement. The partial pressure of a gas is proportional
to the quantity of that gas present in the blood.
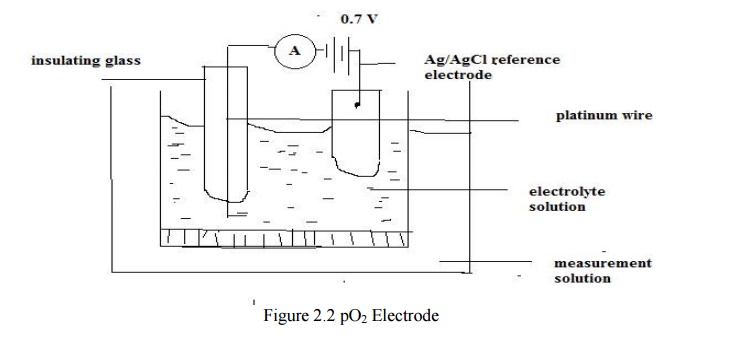
The
platinum wire, which is an active electrode, is embedded in glass for
insulation and only its tip is exposed. It is kept in the electrolyte solution
in which the oxygen is allowed to diffuse. The reference electrode is made up
of silver-silver chloride (Ab/AgCl). A voltage of 0.7 is applied between the
platinum wire and the reference electrode. The negative terminal is connected
to the active electrode through a micro ammeter and the positive terminal is
given to the reference electrode.
Due to
the negative terminal, the oxygen reduction takes place at the platinum
cathode. Finally the oxidation reduction current proportional to the partial
pressure of oxygen diffused into the electrolyte can be measured in the micro
ammeter. The electrolyte is generally scaled in the electrode chamber by means
of a membrane through which the oxygen can diffuse from the blood or sample
solution.
There are
two types of pO2 measurement. They are
I)
Vitro measurement
II)
Vivo measurement
In case
of dark electrode the platinum cathode and the reference electrode is present
in a single unit. This electrode is used for vitro and vivo measurements.
In Vitro Measurements
In this method
the blood sample is taken and the measurement for oxygen saturation is made in
the laboratory. The electrode is placed in the sample blood solution and the pO2
value is determined.
In Vivo Measurements
In this
method the oxygen saturation is determined while the blood is flowing in the
circulatory system. A micro version of the pO2 electrode is placed
at the tip of the catheter so that it can be inserted into various parts of the
heart or circulatory system.
The pO2
measurement also has some disadvantages in it. The reduction process in the
platinum cathode removes a finite amount of the oxygen from the cathode. And
there is a gradual reduction of current with respect to time. However careful
design and proper procedures in modern pO2 electrodes reduce the
errors.
Related Topics