Chapter: Medical Electronics : Bio-Chemical and Non Electrical Parameter Measurement
Colorimeter
COLORIMETER
·
Measures the color concentration of a substance in
a solution by deteecting the color light intensity passing through a sample
containing the substance and a reagent
·
Optical color filters are used to detect the color
wavelength of interest. E.g., urine passes yellow light and absorbs blue and
green
·
Laser LEDs are preferred if their wavelength is
suitable due to purity of the monochromatic color.
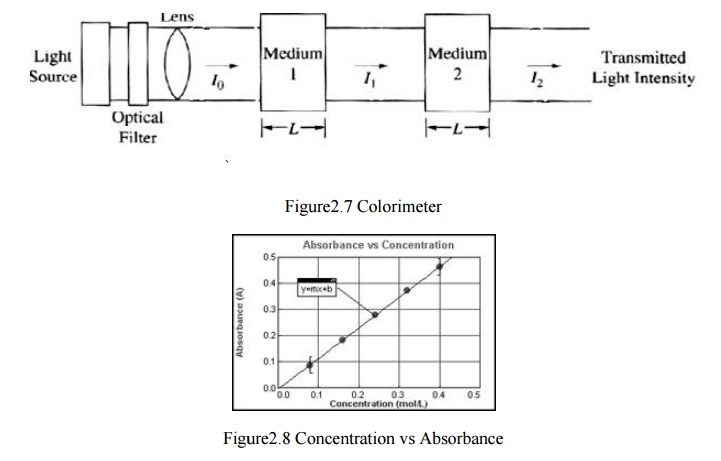
Transmittance
T= I1/I0
* 100%
Absorbance
A= - log I1/ I0
A=log 1/T
If the
path length or concentration increases, the transmittance decreas es and
absorbance increases, a phenomenon expres sed by Beer’s Law.
Absorbtivity
related to the nature of the A=aCL absorbing substance and optical
wavelength (known for a standar d solutionconcentration).
C:
Concentration
L:
Cuvette path length
Related Topics