Chapter: Medical Electronics : Bio-Chemical and Non Electrical Parameter Measurement
Photometer
PHOTOMETER
1. FLAME PHOTOMETER
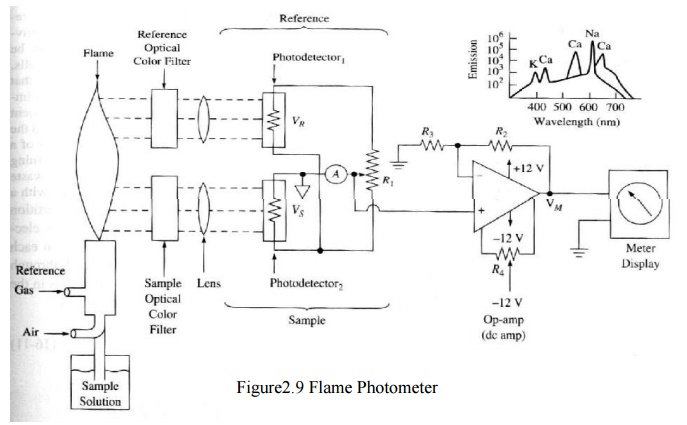
Measures the color intensity of a flame supported by O2 and a specific substance. Sample’s emission of light is measured (rather than the absorbance of light).Typically used to determine the conc. of pure metals and/or Na+, K+, Li+ and Ca++
In this
method, fine droplets of the sample is aspirated into gas flame that burns in a
chimney. A known amount of lithium salt is added to the sample, as a reference.
As a result, red light is emitted by the lithium and yellow and violet beam are
emitted due to sodium and potassium respectively. These diffracted colours are
made to incident on photodiodes. The photo detector circuits consists of a
reverse biased diode in which the current flow increases as intensity of
incident light increases. A calibration potentiometer is used in every channel.
Since the lithium is used as a standard reference, the output of sodium and
potassium channel are calibrated in terms of differences with the known
lithium. The output can be compared with the spectral illustration.
2. SPECTROPHOTOMETER
The
general name given to the group of instruments whose principle of operation is
based on the fact that substances of clinical interest selectively absorb or
emit EM energy (light) at different wavelengths.
·
Depending on the substance being measured, the
wavelength used is typically in the ultraviolet (200-400 nm), visible
(400-700nm) or infrared (700 to 800 nm) range.
·
Spectrophotometer can be used to determine the
entity of an unknown substance, or the concentration of a number of known
substances.
·
The type of source / filters used typically
determines the type of the spectrophotometer.
·
Rays of light bend around sharp corners, where the
amount of bending depends on the wavelength! This results in separation of
light into a spectrum at each line.
·
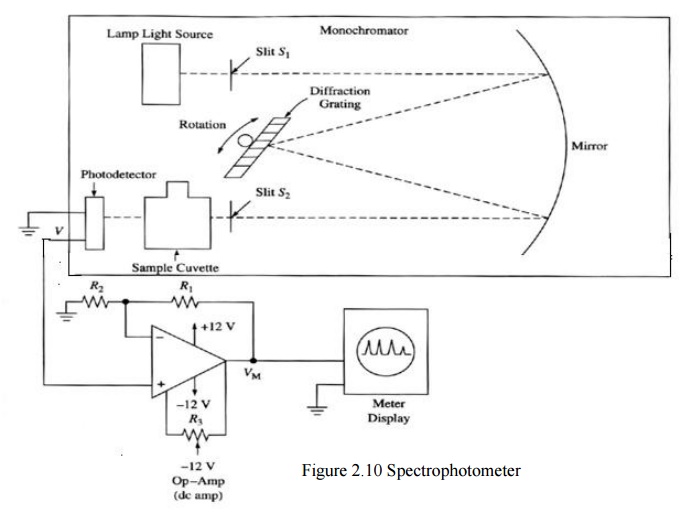
In spectrophotometer, selection filter of
colorimeter is replaced by a monochromator. Monochromatic uses a diffraction
grating G to disperse light from the lamp. Light falls through the slit S0
into its spectral components.
·
Slit S1 is used for selecting a narrow
band of the spectrum which is used to measure the absorption of a sample in the
cuvette.
·
The light from the cuvette is given to photo
detector. It converts light into a electrical signal. This electrical signal is
amplified by using an amplifier. The output from the amplifier is given to
meter which shows absorbance.
· Light absorption is varied when the wavelength is varied. Mirror M is used to reduce the size of the instruments.
Related Topics